|
Introduction
Anaplastic
thyroid carcinoma(ATC) is an aggressive thyroid
malignancy, accounting for 1-4% of thyroid
carcinomas.(1) It typically affects older
individuals in their eighth decade, with a higher
incidence in females.(1) The clinical presentation
often includes acute onset stridor and local pain,
with patients exhibiting regional lymph node
metastasis and recurrent laryngeal nerve
involvement. ATC is known for its propensity for
distant metastasis, particularly to the lungs and
brain. Few patients have a history of
long-standing goiter and an association with
thyrotoxicosis.(2) The median survival of these
patients is extremely bleak, ranging from 1-6
months.(1)
ATC originates from
undifferentiated cells of thyroid follicles,
resulting in aggressive and rapidly progressive
tumors. Prior literature review suggests ATC
arises from pre-existing differentiated thyroid
carcinoma(DTC), constituting up to 23-55% of
cases.(1) ATC has various morphological patterns,
making it challenging to differentiate from
metastatic tumors. The morphological spectrum
includes spindle cell/sarcomatous, epithelioid,
squamous, giant cell, pleomorphic and mixed
patterns.(2) The molecular pathogenesis includes
mutations in BRAF, RAS, CTNNB1, PIK3CA, and TP53
genes.(3) ATC typically exhibit tumor-associated
macrophage(TAMs) infiltration, which accounts for
40 to 70% of the total tumor mass and acts as an
immunosuppressive tumor stroma, contributing to
treatment resistance and poor prognosis.(2)
Previously, ATC was
by default classified as stage IV in the American
Joint Committee on Cancer (AJCC) 7th edition.
However, the 5th edition of World Health
Organization (WHO) now stages it like other
differentiated thyroid carcinomas according to
AJCC 8th edition.(1) Multimodal therapy with a
combination of surgery, external beam radiation
therapy (EBRT), and chemotherapy along with
targeted therapy has shown better overall survival
in ATC.(2)
Material and Methods
A 6-year
retrospective study (July 2018 to July 2024) of
diagnosed cases of ATC in the Department of
Pathology with institutional ethics committee
(IEC) approval. Histopathologically confirmed
cases of ATC were meticulously searched from the
hospital database. Cases with viable tumour tissue
and available slides and blocks were retrieved for
detailed analysis.
The details on the
age, sex, clinical, biochemical parameters like
thyroid function tests, serum calcium, relevant
past history, imaging details, type of excision,
treatment details, metastatic sites involved,
follow up data were collected from patient
electronic medical records. Gross findings,
pathological staging details according to TNM
Classification, AJCC 8th edition was
documented.
Cases lacking
histological confirmation of ATC, unavailability
of data, slides, blocks, non-neoplastic thyroid
lesions, inflammatory conditions, and benign
tumors of thyroid were excluded from the study.
The available
histopathology slides and stained or freshly cut
from retrieved blocks were examined for
histological features like ATC subtype, pattern,
necrosis, mitosis, lymphatic/angioinvasion,
perineural invasion and tumour-infiltrating
lymphocytes (TILs). Other histological parameters
like extrathyroidal extension, adjacent thyroid
findings, other tissue or organ involvement,
margin status, lymph node metastasis and
extra-nodal extension were evaluated. DTC
components were assessed wherever possible.
Representative tumor
foci were marked for tissue microarray(TMA)
excluding slides with necrotic or poorly preserved
tissue. Among the 15 cases of ATC, 12 were
suitable for TMA and underwent BRAFV600E
immunohistochemistry(IHC) using clone IHC600. The
IHC slide was interpreted as positive if >50%
of tumour cells showed intense to moderate
cytoplasmic granular stain and focal if <50%
showed weak cytoplasmic granular staining. The
already available IHC slides of each case were
reviewed with a robust IHC panel of PAX8(MD-50),
TTF1(SPT24), CK(AE1/AE3), Vimentin(SP20),
p53(do-7), p40(Delta NPP), Synaptophysin(EP158),
Chromogranin(EP38), CD56(123C3), CD34(QB-End/10),
CMYC(Y69), Desmin(D33), HMB45(HMB-45), GFAP(GA5),
CEA(CEAm), NapainA(BS10) and BRAF(IHC600). Based
on the hospital records of the patients diagnosed
with ATC, these patients were followed up till the
last hospital visit or until death.
The collected data
were entered in the Microsoft Excel 2016 and
analysed with IBM SPSS Statistics for Windows,
Version 29.0.(Armonk, NY: IBM Corp).To describe
about the data descriptive statistics, T test was
used to compare means, and Chi-square test was
employed to study the association between the
categorical variables. To find the Survival
analysis the Kaplan Meier Curve with Log-rank
method were used. In the above statistical tools,
the probability value .05 is considered as
significant level.
Results
This retrospective
study spanning six years analysed fifteen
histologically confirmed cases of ATC. The age
ranged from 48-82 years, with a mean of 66.1 years
and a male to female ratio of 1:2. The most
prevalent clinical presentation was anterior neck
swelling(n=12, 80%), followed by odynophagia(n=7,
46.6%), voice change(n=7, 46.6%),
breathlessness(n=4, 26.6%) and stridor(n=3, 20%).
Symptoms ranged from 2 days-8 months. 33.3% (n=5)
had a long-standing goiter (20-25 years) with
rapid progression. One patient reported biomass
exposure for 20 years. 73.3%(n=11) were euthyroid,
20%(n=3) hypothyroid and 6.6%(n=1) hyperthyroid
with toxic multinodular goiter. 73.3%(n=11) had
leukocytosis (>10000 cells/cu mm). Serum
calcium levels were within normal limits in all
cases.
Radiologically,
60%(n=9) revealed distant metastasis at diagnosis,
primarily affecting lung(n=8, 53.3%), followed by
bone(vertebrae and rib)(n=4, 26.6%), pancreas(n=1,
6.6%) and 26.6% had multiple sites of distant
metastasis.
80%(n=12) showed
locally advanced lesions involving strap muscle,
thyroid cartilage, prevertebral space, recurrent
laryngeal nerve, trachea, larynx, and carotid
vessel. Cervical lymph node metastasis was noted
in 26.6%(n=4) cases. Tumor size ranged from 2-13cm
with a mean of 7.1cm, presenting as either
unifocal(n=9, 60%) or multifocal tumors(n=6, 40%).
The clinicopathological features are summarized in
Table 1.
|
Table 1: Demographic and
case details of Anaplastic thyroid
carcinoma.
|
|
Variables
|
N
|
Percentage
|
P value
|
|
Total number of cases
|
15
|
|
|
|
Age (48-82 years)
|
|
|
|
|
<60
|
3
|
20.00
|
0.759
|
|
>60
|
12
|
80.00
|
|
Gender
|
|
|
|
|
Male
|
5
|
33.33
|
0.293
|
|
Female
|
10
|
66.67
|
|
Duration (2 days- 8 months)
|
|
<1month
|
8
|
53.33
|
0.139
|
|
>1month
|
7
|
46.67
|
|
Tumour size (2-13cm)
|
|
<5cm
|
3
|
20.00
|
0.596
|
|
>5cm
|
12
|
80.00
|
|
Thyroid function test
|
|
Euthyroid
|
11
|
73.33
|
0.368
|
|
Hypothyroid
|
3
|
20.00
|
|
Hyperthyroid
|
1
|
6.67
|
|
|
Total leukocyte count(cells/mm)
|
|
>10000 cells/mm
|
11
|
73.33
|
0.291
|
|
<10000 cells/mm
|
4
|
26.67
|
|
Differentiated thyroid carcinoma
|
|
Associated with PTC
|
4
|
26.67
|
0.620
|
|
Associated with FTC
|
3
|
20.00
|
|
Stage
|
|
pT3
|
7
|
46.67
|
0.201
|
|
pT4a
|
6
|
40.00
|
|
pT4b
|
2
|
13.33
|
|
Follow up (Till last follow up)
|
|
Lost to follow up
|
1
|
6.67
|
0.691
|
|
Alive
|
2
|
13.33
|
|
Dead
|
12
|
80.00
|
Grossly, tumours appeared solid to cystic,
exhibiting extensive necrosis, haemorrhage, and
gross extrathyroidal invasion(Figure 1).
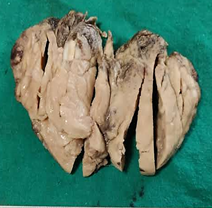
|
| Figure
1: Anaplastic thyroid carcinoma
showing large, fleshy, gray-white tumor
replacing the entire lobe. |
Frozen section was
performed on 6 patients on an emergency basis in
view of stridor and was diagnosed as ATC in 5
cases, while one was deemed negative for
malignancy. Histopathology revealed varied
morphological patterns like epithelioid(n=5,
33.3%)(Figure 2A), sarcomatoid(n=5, 33.3%)(Figure
2B), squamous(n=4, 26.6%)(Figure 2C) and
pleomorphic/rhabdoid(n=1, 6.6%)(Figure 2D).

|
| Figure
2: A. Anaplastic thyroid
carcinoma, Epithelioid subtype(200x, H
& E). B. Anaplastic thyroid carcinoma,
Sarcomatoid subtype(200x, H & E). C.
Squamous cell carcinoma(200x, H & E).
D. Anaplastic thyroid carcinoma, Rhabdoid
subtype(200x, H & E). |
Mixed configurations
of epithelioid and spindle pattern(n=3,
20%)(Figure 3A) and spindle with pleomorphic
features (n=2, 13.3%) were identified. Other
findings included osteoclast-like giant cells(n=1,
6.6%) (Figure 3B), myxoid stroma(n=1, 6.6%)(Figure
3C) and osteoid-like matrix, reminiscent of
osteosarcoma(n=1, 6.6%)(Figure 3D).

|
| Figure
3: A. Anaplastic thyroid
carcinoma, mixed epithelioid and spindle
subtype(200x, H & E). B. ATC with
osteoclast like giant cell rich
areas(200x, H & E). C. Sarcomatoid ATC
with prominent myxoid stroma(200x, H &
E). D. ATC with osteoid-like matrix
mimicking osteosarcoma(200x, H & E).
|
Coexistence of ATC
with differentiated thyroid carcinoma was observed
in 46.6% (n=7) of cases. Of these, four cases
(26.6%) were associated with papillary thyroid
carcinoma (PTC) (Figure 4A), and three cases (20%)
with follicular thyroid carcinoma (FTC) (Figure
4B).

|
| Figure
4: A. Squamous cell
carcinoma(lower left) with coexisting
classic Papillary thyroid carcinoma(upper
right) (200x, H & E) and inset showing
p40 positivity. B. ATC with follicular
thyroid carcinoma(200x, H & E). |
Among the subtypes,
13.3% of squamous ATC were accompanying a PTC
precursor. The epithelioid variant of ATC was
associated with both PTC (n=2) and FTC (n=2)
precursors. One case of pure sarcomatoid ATC
showed an FTC precursor. The association of the
clinicopathologic features with ATC subtypes are
given in Table 2.
|
Table 2: Association of
clinicopathological characteristics and
morphological subtypes of 15 ATC.
|
|
Variables
|
Epithelioid n(%)
|
Squamous n(%)
|
Sarcomatoid n(%)
|
Pleomorphic/ Rhabdoid n(%)
|
Chi square value
|
P value
|
|
Total number of cases(n=15)
|
5(33.3%)
|
4(26.6%)
|
5(33.3%)
|
1(6.6%)
|
|
|
|
Age (48-82 years)
|
1.105
|
0.775
|
|
<60(n=3)
|
1(6.6%)
|
0
|
2(13.3%)
|
0
|
|
>60(n=12)
|
4(26.6%)
|
4(26.6%)
|
3(20%)
|
1(6.6%)
|
|
Gender
|
0.7985
|
0.849
|
|
Male(n=5)
|
2(13.3%)
|
0
|
2(13.3%)
|
1(6.6%)
|
|
Female(n=10)
|
3(20%)
|
4(26.6%)
|
3(20%)
|
0
|
|
Tumour infiltrating lymphocytes
(TILs)
|
3.7542
|
0.289
|
|
Low(<5%)(n=7)
|
2(13.3%)
|
0
|
4(26.6%)
|
1(6.6%)
|
|
High(>6%)(n=8)
|
3(20%)
|
4(26.6%)
|
1(6.6%)
|
0
|
|
BRAF IHC
|
3.6
|
0.308
|
|
Positive(n=7)
|
4(26.6%)
|
2(13.3%)
|
0
|
1(6.6%)
|
|
Negative(n=8)
|
1(6.6%)
|
2(13.3%)
|
5(33.3%)
|
0
|
|
Differentiated thyroid carcinoma
|
0.2444
|
0.970
|
|
Associated with PTC(n=4)
|
2(13.3%)
|
2(13.3%)
|
0
|
0
|
|
Associated with FTC(n=3)
|
2(13.3%)
|
0
|
1(6.6%)
|
0
|
|
Stage
|
3.375
|
0.76
|
|
pT3(n=7)
|
1(6.6%)
|
2(13.3%)
|
4(26.6%)
|
0
|
|
pT4(n=8)
|
4(26.6%)
|
2(13.3%)
|
1(6.6%)
|
1(6.6%)
|
|
Distant metastasis at
presentation (n=9)
|
3(20%)
|
2(13.3%)
|
4(26.6%)
|
0
|
1.0667
|
0.785
|
|
Absence of distant metastasis at
presentation
|
2(13.3%)
|
2(13.3%)
|
1(6.6%)
|
1(6.6%)
|
|
Follow up (Till last follow up)
|
2.385
|
0.881
|
|
Lost to follow up(n=1)
|
1(6.6%)
|
0
|
0
|
0
|
|
Alive(n=2)
|
0
|
2(13.3%)
|
0
|
0
|
|
Dead(n=12)
|
4(26.6%)
|
2(13.3%)
|
5(33.3%)
|
1(6.6%)
|
While DTC components
were identifiable in larger excision specimens,
their presence often went undetected in needle
biopsies, underscoring the limitations of these
approaches. Adjacent thyroid revealed lymphocytic
thyroiditis(n=5, 33.3%) and multinodular
goiter(n=5, 33.3%). A hallmark feature was the
presence of neutrophilic inflammatory infiltrate.
Extensive areas of necrosis, atypical mitosis,
angioinvasion was observed in all cases.
High TILs(>10%)
were noted in epithelioid(20%) and squamous(26.6%)
ATC, while low TILs(<5%) in sarcomatoid(26.6%)
and pleomorphic/rhabdoid(6.6%) ATC.
Immunohistochemistry(IHC)
revealed strong, nuclear expression of PAX8 in
66.6%(n=10)(Figure 5A) and negative in sarcomatoid
ATC. TTF1 showed focal weak nuclear
positive(53.3%, n=8)(Figure 5A[Inset]), 73.3%
expressed p53 mutant type diffuse nuclear
positivity(Figure 5B) with one case showed null
pattern. P40 was positive in all squamous ATC.
Sarcomatoid ATC expressed vimentin, desmin and
focal to negative CK. Napsin A was performed and
all were negative. 53.3%(n=8) expressed BRAF V600E
by IHC. Among these, four PTC-associated ATC and
one pure pleomorphic/rhabdoid ATC showed diffuse
intense BRAF positivity(Figure 5C). Focal BRAF
staining was seen in two epithelioid ATC(Figure
5D).

|
| Figure
5: A. Immunohistochemistry
reveals diffuse nuclear expression of
PAX8(200x), absence of TTF1 with positive
internal control in thyroid
follicles[Inset]. B. p53 mutant
pattern(200x). C. Diffuse cytoplasmic BRAF
expression(200x). D. Focal weak
cytoplasmic staining of BRAF IHC(200x).
|
Immunohistochemistry findings are as highlighted
in Table 3.
|
Table 3 Illustration of
the IHC expression. (+) Positive staining,
(-) Negative staining, (F) Focal staining,
(ND) Not done, IHC Immunohistochemistry,
*p53 Null pattern of staining.
|
|
Case
|
Histomorphological pattern
|
IHC
|
|
|
PAX8
|
TTF1
|
CK
|
P53
|
P40
|
BRAF
|
NapsinA
|
|
1
|
Epithelioid
|
+
|
ND
|
+
|
+
|
ND
|
+ (F)
|
-
|
|
2
|
Squamous cell carcinoma
|
+
|
+(F)
|
ND
|
ND
|
+
|
-
|
-
|
|
3
|
Pleomorphic/Rhabdoid
|
+
|
+
|
+
|
+
|
-
|
+
|
-
|
|
4
|
Epithelioid
|
+
|
+(F)
|
+
|
-
|
ND
|
+(F)
|
-
|
|
5
|
Epithelioid
|
+
|
-
|
+(F)
|
+
|
ND
|
+
|
-
|
|
6
|
Squamous
|
+
|
-
|
+
|
-
|
+
|
-
|
-
|
|
7
|
Sarcomatoid
|
-
|
-
|
-
|
+
|
ND
|
-
|
-
|
|
8
|
Sarcomatoid
|
-
|
+(F)
|
+(F)
|
+
|
ND
|
-
|
-
|
|
9
|
Epithelioid
|
+
|
+(F)
|
+
|
+
|
-
|
-
|
-
|
|
10
|
Epithelioid
|
+
|
+(F)
|
+
|
+
|
ND
|
+
|
-
|
|
11
|
Sarcomatoid
|
-
|
+(F)
|
-
|
+*
|
-
|
-
|
-
|
|
12
|
Squamous
|
+
|
+(F)
|
+
|
ND
|
+
|
+
|
-
|
|
13
|
Sarcomatoid
|
-
|
-
|
+
|
+
|
ND
|
-
|
-
|
|
14
|
Squamous
|
+
|
+
|
ND
|
+
|
+
|
+
|
-
|
|
15
|
Sarcomatoid
|
-
|
-
|
-
|
+
|
ND
|
-
|
-
|
Only one patient
received multimodal treatment with chemotherapy
and radiotherapy post radical resection. Financial
constraints precluded multimodal therapies,
limiting options to palliative care in 14
patients. On follow-up, two patients were alive
with disease(AWD), 12 patients died of
disease(DOD) and one patient was lost on
follow-up(LOF). The patients were followed up for
a duration of 1 month-18 months. Treatment
characteristics are illustrated in Table 4.
Two(13.3%) patients
with squamous ATC and associated PTC precursor
with BRAF mutation were AWD at 7 months and 18
months of last follow up respectively. Among
these, one patient received multimodal treatment
had local recurrence with lung metastasis and AWD
at 18 months of follow up. The median overall
survival of 15 cases was one month.
|
Table 4: Treatment
characteristics with median survival of
ATC patients.
|
|
Treatment received
|
Number of patients
|
Median survival in days
|
P value
|
Follow up
|
|
Radical resections
|
0.65
|
|
|
Total laryngectomy, total thyroidectomy,
bilateral cervical neck node dissection
|
1
|
212
|
Alive
|
|
Total thyroidectomy
|
3
|
60
|
Dead
|
|
Total thyroidectomy + radiotherapy and
chemotherapy(CT+RT)
|
1
|
547
|
Alive
|
|
Lobectomy with neck dissection
|
1
|
60
|
Dead
|
|
Non-radical resections
|
|
|
Debulking procedure
|
3
|
30
|
Dead
|
|
Biopsy and tracheostomy
|
3
|
30
|
Dead
|
|
Biopsy without tracheostomy
|
3
|
60
|
Dead
|
The survival
analysis using the Kaplan-Meier curve and Log-rank
method compared with age <60 and >60 years
resulted in a p-value of 0.468, while between
tumour size <5cm and >5cm yielded a p-value
of 0.696 indicating no statistical
significance(Figure 6A & 6B).
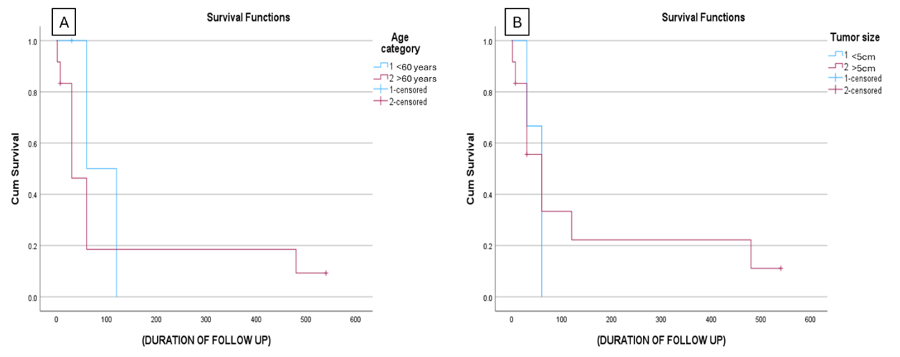
|
| Figure
6: Survival curve of ATC
patients for: (a) Age and (b) Tumour size.
|
Discussion
ATC is a highly
aggressive, undifferentiated thyroid malignancy
exhibits endpoint of progression from DTC.(4) In
the current study 46.6% of cases had associated
DTC. According to McIver et al., the progression
from DTC to ATC spans 2-32 years, with a mean
duration of 9.5 years.(5)
ATC accounts for
1-4% of all thyroid cancers worldwide and 1% in
the USA and is responsible for 14-39% of thyroid
cancer-related deaths.(1) ATC affects the
geriatric group peaking in the eighth decade.(6,7)
However, we observed a slightly younger cohort
with a mean age of 66.1 years(7th decade).
Literature review suggests a female preponderance
consistent with our findings with male-to-female
ratio of 1:2.(5,6,8)
ATC lacks a clearly
defined clinical presentation, often manifesting
with neck swelling and compressive symptoms
involving the trachea, esophagus, and involvement
of recurrent laryngeal nerve, similar to this
study.(1) Long-standing goiter of 20-25 years,
experience sudden progression within a month, were
observed in 33.3% of cases in our cohort,
mirroring findings by Pradhan et al.(9).
Cervical lymph node metastasis has been reported
in 29-64% in other studies(10), with the current
study observing it in 26.6% of cases. Lung is the
most common sites of distant metastasis,
consistent with findings in literature.(9,11)
While previous
studies reported associations with thyrotoxicosis,
goiter, and paraneoplastic syndrome, our cohort
was primarily euthyroid with leukocytosis(73.3%).
Notably, 60% of patients were diagnosed with stage
IVC disease, while 33.3% were in stage IVB,
aligning closely with observations by Jannin and
Zhang et al.(2,12)
ATC demonstrates
diverse histology with heterologous elements like
osteoid and cartilaginous components.(1,10)
Similar to our findings, PTC forms the most
associated DTC as shown in a study of 360 ATC
cases.(13) It can also be seen with FTC and
oxyphilic-Hürthle cell carcinoma.(14) Also, a
significant association is seen between the tall
cell variant of papillary carcinoma and squamous
ATC as demonstrated in other studies.(13,15,16) In
contrast, sarcomatoid ATC is more closely related
to FTC and often harbors RAS mutations.(15)
Sarcomatoid histological type is associated with
the highest overall mortality, with all
sarcomatoid cases in this study presenting at a
higher stage with distant metastases. The WHO 5th
Edition now recognizes squamous cell carcinoma
(SCC) of the thyroid as a morphological subtype of
ATC.(17) The molecular and clinical behavior
reveal overlapping features between SCC and ATC,
originating from follicular cells and expressing
BRAFV600E, TTF1, and PAX8 with poor prognosis.(18)
According to Chen et
al, BRAF-mutated ATC constitutes 36% and is
associated with a high frequency of DTC,
particularly PTC similar to the current study and
shows a higher association with squamous
patterns.(14) BRAF has therapeutic benefits in
ATC. Therefore, BRAFV600E mutation testing is
suggested in all cases. FDA has approved
dabrafenib and trametinib (targeting BRAF and
MEK1/2) for patients with BRAFV600E-mutant
ATC(15,19)
We also found high
TILs (>10-20%) in 53.3% of ATC, mainly in
epithelioid(20%) and squamous(26.6%) subtype, in
contrast to low TILs(<5%) in sarcomatoid ATC.
The significance of TILs in ATC is still under
investigation. A positive correlation between
PD-L1 and BRAFV600E was seen along with
association of epithelioid pattern exhibiting
increased PD-L1 compared to sarcomatoid pattern of
ATC, like the findings noticed by other
researchers.(20–22) Immunotherapy, particularly
spartalizumab (anti-PD-1 antibody), has shown
promising results by Capdevila et al(23)
PAX8 is a sensitive
marker compared to TTF1 and shows increased
expression with epithelioid and squamous ATC,
making it invaluable for distinguishing ATC from
metastatic lung carcinoma. According to Nonaka et
al, PAX8 expression was observed in 79% of ATC,
compared to TTF-1 in 18%.(24) We observed PAX8 in
66.6% and TTF1 in 53.3%. In the present study,
NapsinA was negative in all cases, however, Wu et
al showed 11.1% of Napsin A expression in ATC
aiding differentiation from metastatic lung
carcinoma when combined with thyroid markers.(25)
The diagnosis of ATC hinges on identifying DTC
components, establishing thyroid as the primary
tumour site, exclusion of metastasis or direct
invasion from adjacent structures. Differentiating
high-grade non-anaplastic thyroid carcinoma relies
on thyroglobulin expression and lack of anaplasia.
According to prior
literature, old age(>70 years), males,
leukocytosis, extrathyroidal extension with
distant metastasis at presentation are poor
prognostic features.(6,11,12) Contrary to previous
notions, age, tumour size and leukocytosis did not
impact prognosis in the current study, similar to
findings by Masui et al(26). Xu et al showed that
the site of ATC, morphologic features, necrosis,
mitoses and distant metastasis, was not associated
with outcomes.(13) Our study revealed
a better outcome with BRAF mutant squamous ATC,
suggesting that this subset may derive particular
benefit from targeted treatments. However, no
statistically significant associations were
observed between age, tumour size or morphological
subtypes with survival.
One patient who
received surgical intervention with multimodal
treatment survived for 7 months, proving that
incorporation of multimodal therapy including
surgical intervention has shown promise in
prolonging survival.(27) Patients undergoing total
thyroidectomy demonstrate superior outcomes
compared to partial resections, although lymph
node dissection does not influence survival.(6)
Despite surgical intervention, ATC prognosis
remains grim, with only 20% of patients surviving
beyond one year.(10) However, the limited survival
time and poor statistical data on ATC underscore
the need for more research and improved treatment
strategies.
Conclusion
This six-year
retrospective analysis highlights the aggressive
nature of ATC, often diagnosed at an advanced
stage with frequent metastasis. The patients
presented in seventh decade with acute
manifestations. ATC often arises from DTC,
particularly papillary thyroid carcinoma in
epithelioid and squamous subtypes with expression
of BRAF. BRAF-mutated squamous ATC has prolonged
survival in this cohort. Application of targeted
therapy based on BRAFV600E status may aid in
prognostication and improve outcomes in selected
patients. Future research must focus on molecular
risk stratification, integration of targeted
therapy and immunotherapy into multimodal
treatment strategies.
Limitations of the Study
The study faced several limitations, including a
small sample size, retrospective nature, lack of
molecular testing beyond IHC, inherent limitations
of core needle biopsies, which fail to capture
crucial elements such as DTC components and
staging parameters. The statistical analysis was
attempted; however, no significant values were
obtained in view of small sample size.
Competing interests
The authors declare that they have no competing
interests.
Ethics approval and consent
to participate
The study was approved by Institutional ethical
committee of Kasturba Medical College, Manipal
(IEC1 – 404). Since it was retrospective study,
consent from the participants was waived.
References
- Abe I, Lam KY. Anaplastic thyroid carcinoma:
Updates on WHO classification,
clinicopathological features and staging. Histol
Histopathol. 2021 Mar;36(3):239–48.
- Jannin A, Escande A, Al Ghuzlan A, et al.
Anaplastic thyroid carcinoma: an update. Cancers.
2022 Feb 19;14(4):1061.
- Volante M, Lam AK, Papotti M, et al. Molecular
pathology of poorly differentiated and
anaplastic thyroid cancer: what do pathologists
need to know?. Endocrine Pathology. 2021
Mar;32:63-76.
- Basolo F, Macerola E, Poma AM, et al. The 5th
edition of WHO classification of tumors of
endocrine organs: changes in the diagnosis of
follicular-derived thyroid carcinoma. Endocrine.
2023 Jun;80(3):470-6.
- McIver B, Hay ID, Giuffrida DF, et al.
Anaplastic thyroid carcinoma: a 50-year
experience at a single institution. Surgery.
2001 Dec 1;130(6):1028-34.
- Lin B, Ma H, Ma M, et al. The incidence and
survival analysis for anaplastic thyroid cancer:
a SEER database analysis. American Journal
of Translational Research. 2019 Sep
15;11(9):5888.
- Kim TY, Kim KW, Jung TS, et al. Prognostic
factors for Korean patients with anaplastic
thyroid carcinoma. Head & Neck: Journal for
the Sciences and Specialties of the Head and
Neck. 2007 Aug;29(8):765-72.
- Kanteti AP, Ghose J, Patil VM, et al.
Anaplastic cancer: Our experience. Indian
Journal of Surgical Oncology. 2022
Dec;13(4):789-96.
- Pradhan R, Agarwal A, Lal P, et al.
Clinico-pathological profile of anaplastic
thyroid carcinoma in an endemic goiter area. Indian
Journal of Endocrinology and Metabolism. 2018
Nov 1;22(6):793-7.
- Deeken-Draisey A, Yang GY, Gao J, et al.
Anaplastic thyroid carcinoma: an epidemiologic,
histologic, immunohistochemical, and molecular
single-institution study. Human Pathology. 2018
Dec 1;82:140-8.
- Sugitani I, Miyauchi A, Sugino K, et al.
Prognostic factors and treatment outcomes for
anaplastic thyroid carcinoma: ATC Research
Consortium of Japan cohort study of 677
patients. World Journal of Surgery. 2012
Jun;36(6):1247-54..
- Zhang K, Wang X, Wei T, et al. Comparative
study between poorly differentiated thyroid
cancer and anaplastic thyroid cancer: real-world
pathological distribution, death attribution,
and prognostic factor estimation. Frontiers
in Endocrinology. 2024 Mar 13;15:1347362.
- Xu B, Fuchs T, Dogan S, et al. Dissecting
anaplastic thyroid carcinoma: a comprehensive
clinical, histologic, immunophenotypic, and
molecular study of 360 cases. Thyroid. 2020
Oct 1;30(10):1505-17.
- Chen TY, Lorch JH, Wong KS, et al.
Histological features of BRAF V600E‐mutant
anaplastic thyroid carcinoma. Histopathology.
2020 Aug;77(2):314-20.
- Gu H, Wang J, Ran W, et al. Anaplastic and
poorly differentiated thyroid carcinomas:
genetic evidence of high‐grade transformation
from differentiated thyroid carcinoma. The
Journal of Pathology: Clinical Research. 2024
Mar;10(2):e356.
- Ragazzi M, Ciarrocchi A, Sancisi V, et al.
Update on anaplastic thyroid carcinoma:
morphological, molecular, and genetic features
of the most aggressive thyroid cancer. International
Journal of Endocrinology. 2014;2014(1):790834.
- Juhlin CC, Mete O, Baloch ZW. The 2022 WHO
classification of thyroid tumors: novel concepts
in nomenclature and grading. Endocrine-related
Cancer. 2023 Feb 1;30(2).
- Lam AK. Squamous cell carcinoma of thyroid: a
unique type of cancer in World Health
Organization Classification. Endocrine-Related
Cancer. 2020 Jun 1;27(6):R177-92.
- da Silva TN, Rodrigues R, Saramago A, et al.
Target therapy for BRAF mutated anaplastic
thyroid cancer: a clinical and molecular study.
European Journal of Endocrinology. 2023
Jan 10;188(1):31-8.
- Agarwal S, Jung CK, Gaddam P, et al. PD-L1
expression and its modulating factors in
anaplastic thyroid carcinoma: a
multi-institutional study. The American
Journal of Surgical Pathology. 2024 Oct
1;48(10):1233-44.
- Zwaenepoel K, Jacobs J, De Meulenaere A, et
al. CD 70 and PD‐L1 in anaplastic thyroid
cancer–promising targets for immunotherapy. Histopathology.
2017 Sep;71(3):357-65.
- Cameselle-García S, Abdulkader-Sande S,
Sánchez-Ares M, et al. PD-L1 expression and
immune cells in anaplastic carcinoma and poorly
differentiated carcinoma of the human thyroid
gland: A retrospective study. Oncology
Letters. 2021 Jul;22(1):553.
- Capdevila J, Wirth LJ, Ernst T, et al. PD-1
blockade in anaplastic thyroid carcinoma. Journal
of Clinical Oncology. 2020 Aug
10;38(23):2620-7.
- Nonaka D, Tang Y, Chiriboga L, et al.
Diagnostic utility of thyroid transcription
factors Pax8 and TTF-2 (FoxE1) in thyroid
epithelial neoplasms. Modern Pathology. 2008
Feb 1;21(2):192-200.
- Wu J, Zhang Y, Ding T, et al. Napsin A
expression in subtypes of thyroid tumors:
comparison with lung adenocarcinomas. Endocrine
Pathology. 2020 Mar;31:39-45.
- Masui T, Uemura H, Ota I, et al. A study of 17
cases for the identification of prognostic
factors for anaplastic thyroid carcinoma. Molecular
and Clinical Oncology. 2021 Jan;14(1):1.
- Song T, Chen L, Zhang H, et al. Multimodal
treatment based on thyroidectomy improves
survival in patients with metastatic anaplastic
thyroid carcinoma: a SEER analysis from 1998 to
2015. Gland Surgery. 2020 Oct;9(5):1205.
|



















