|
Introduction
Achalasia
is a primary esophageal motility disorder
characterized by the failure of the lower
esophageal sphincter (LES) to relax in response to
swallowing, along with weak to absent esophageal
peristalsis. This disorder is uncommon, though
well documented in literature for over 300 years
(1), and causes a significant impairment of eating
ability, thereby adversely impacting the quality
of life (2).
The
pathophysiological basis of this disorder include
a loss of inhibitory function affecting the
esophageal myenteric nerves which result in loss
of esophageal peristalsis and inadequate
relaxation of the lower esophageal sphincter
(LES), thereby resulting in a functional
obstruction (2).Rarely, in advanced cases, the
condition may manifest as a “sigmoid esophagus,”
which is a term that has been coined to describe
the dilation and distortion of the cervical
esophagus (3).
In this report, we
present one such case of a 36-year-old male in
whom sigmoid esophagus was successfully diagnosed
and then managed by a minimally invasive surgical
approach uneventfully. The case is presented
because of the rare nature of this disorder.
Case Presentation
A 36-year-old male
reported with persistent burning sensation in the
chest, difficulty in swallowing food and
regurgitation of undigested food, off and on, for
more than a year. He had been consuming
over-the-counter H2 blockers and proton pump
inhibitors but achieving partial relief only. He
had no significant past medical or surgical
history. He was a non-smoker and had no
addictions.
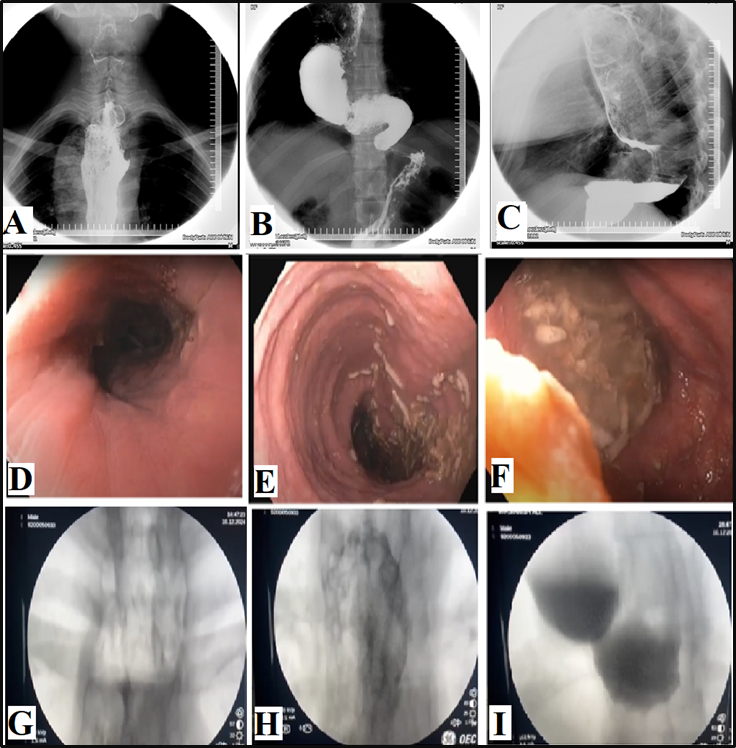
A barium
esophagogram (Figures A, B, and C) revealed a
significantly dilated esophagus with tapering of
the distal esophagus. Esophageal mucosa displayed
irregular distortion with focal areas of
hypertrophy, but no definite pouch or diverticula
was noted. Incomplete LES relaxation was
demonstrated that was not coordinated with
esophageal contraction, followed by uncoordinated,
non-propulsive, tertiary contractions.
Furthermore, there was a failure of normal
peristalsis to clear the esophagus of barium when
the patient lay in the recumbent position, with no
identification of primary waves. There were no
persistent filling defects, abnormal shouldering,
nor any evidence of hiatus hernia.
Esophagogastroduodenoscopy
(EGD) revealed a dilated esophagus with a very
tight lower esophageal sphincter (Figures D, E,
and F). No ulcerative or mass lesion was seen. The
findings were deemed to be consistent with
achalasia cardia. The patient was attached to the
services of a tertiary care facility with
board-certified upper gastrointestinal surgeons.
An axial CT scan of the chest with contrast was
advised, which revealed marked dilatation of the
entire esophagus (maximum 87 mm) down to the
gastroesophageal junction with stagnant food and
fluid levels. The entire dilated esophagus
appeared folded with diffuse wall thickening with
mass effect upon the related mediastinum
structures and lung parenchyma. After proper
counselling, laparoscopic Heller’s myotomy (LHM)
procedure was undertaken as a definitive
management option. The postoperative phase was
uneventful. At six months after surgery, the
patient was asymptomatic and highly satisfied with
the outcomes. A Gastrografin esophagogram study
was undertaken under fluoroscopic guidance (Figure
1 G, H, I). There was evidence of a dilated
esophagus with a tortuous and dilated lower third
of the esophagus with minimal pooling of contrast
in its tortuous segment and decreased peristalsis.
However, there was free passage of contrast into
the stomach and small bowel loops. No gross
filling defect or esophageal web was documented.
|
| Figure
1: A, B, C - Preoperative Barium
esophagogram; D, E, F – Preoperative
esophagogastroduodenoscopy; G, H, I –
Postoperative (6 months) Gastrografin
esophagogram study, undertaken under
fluoroscopic guidance. |
Discussion
Achalasia is a very
rare disorder, with an incidence and a prevalence
of about 1 per 100000 population and 10 per
100000, respectively. The disease is found across
all the races and equally in both the genders. The
patients typically present between the second and
the fifth decade of life, though 2%-5% of cases do
occur in the paediatric age group (4).
The successful
transport of food from the mouth to the stomach,
while preventing its reflux from the stomach, is a
result of the coordinated peristalsis in the
pharynx and esophagus along with the relaxation of
the upper and lower esophageal sphincters (LES),
brought about by the modulatory action of the
parasympathetic excitatory and inhibitory pathways
(myenteric plexus) that innervate the smooth
muscles of the lower esophageal sphincter.
Patients with achalasia lack the inhibitory
ganglion cells while retaining the normal
excitatory neurons and thence suffer from an
imbalance of inhibitory and excitatory
neurotransmission with a resultant non-relaxed
hypertensive LES (3). Functional obstruction
ensues, and gradually the esophagus dilates,
leading to uncoordinated aperistalsis and
worsening obstructive symptoms (2,3), as is
evident in the imaging studies (Figure 1) of our
presented case report.
The majority of
patients with achalasia present with dysphagia
and/or regurgitation. Other symptoms include chest
discomfort, nocturnal cough, heartburn, hiccups,
and weight loss. When achalasia is suspected, a
barium esophagogram (barium swallow) is conducted,
which reveals the smooth tapering of the lower
esophagus to a “bird’s beak” appearance, with
dilatation of the proximal esophagus, lack of
peristalsis during fluoroscopy, and an air-fluid
level (5). In advanced disease, a sigmoid-like
appearance of the esophagus may be visible, as was
seen in the images recorded in the presented case
(Figure 1 A-C). Esophagogastroduodenoscopy (EGD)
is recommended in all patients presenting with
dysphagia to exclude any neoplastic lesions
involving the esophagus (6). EGD otherwise has low
accuracy in the diagnosis of achalasia and may not
reveal any abnormal normal findings in the earlier
stages of the disease. In advanced cases, the
esophagus may appear dilated, tortuous, and
atonic, with retained food contents, as was
documented in our presented case (Figure 1 D, E,
F). High-resolution esophageal manometry (HRM) is
a sensitive test for the diagnosis of achalasia
and reveals an increase in pressure of the LES
with incomplete relaxation in response to
swallowing and, in most cases, a lack of
coordinated peristalsis in the lower esophagus
(7). Manometric findings can aid in the
determination of prognosis and appropriate
treatment modalities.
Once malignancy has
been ruled out, management aims at easing the
symptoms of achalasia by decreasing the outflow
resistance through nonsurgical or surgical
modalities (8-9). The nonsurgical options are
effective in early stages and include
pharmacotherapy, endoscopic botulinum toxin
injection, and pneumatic dilatation, whereas the
surgical options include laparoscopic Heller
myotomy (LHM) and peroral endoscopic myotomy
(POEM). In our case, LHM was conducted
uneventfully, and the patient was symptom-free at
6 months follow-up after surgery. In LHM, the
circular muscle fibers running across the lower
esophageal sphincter are cut, leading to
relaxation (9) . LHM can potentially cause
uncontrolled gastroesophageal reflux, so it
typically pairs with an anti-reflux procedure
(partial fundoplication). The clinical success
rate of LHM is reported to be high (76 to 100%) at
three years, with a low mortality rate of 0.1%.
Even after successful treatment, the patients
require long-term follow-up because all available
treatments are palliative, making recurrences
common, and secondly, there is a small risk of
malignancy (7-10).
Conclusion
Sigmoid esophagus is
a manifestation of an advanced stage of achalasia.
It is rare in occurrence. After ruling out
malignancy, laparoscopic Heller myotomy is an
effective and safe management option.
Postoperatively, the patients require long-term
follow-up for early detection of recurrence,
reflux, or malignancy.
Acknowledgements
The patient provided
us with his case history and photos for academic
purposes, including publishing in a peer-reviewed
journal, for which the authors are grateful.
References
- Kraichely RE, Farrugia G. Achalasia:
physiology and etiopathogenesis. Dis
Esophagus. 2006;19(4):213-23.
- Furuzawa-Carballeda J, Torres-Landa S,
Valdovinos MÁ, Coss-Adame E, Martín Del Campo
LA, Torres-Villalobos G. New insights into the
pathophysiology of achalasia and implications
for future treatment. World J Gastroenterol.
2016 Sep 21;22(35):7892-907.
- Vallejo C, Gheit Y, Nagi TK, Suarez ZK, Haider
MA. A Rare Case of Achalasia Sigmoid Esophagus
Obstructed by Food Bolus. Cureus. 2023
Sep 19;15(9): e45567.
- Wadhwa V, Thota PN, Parikh MP, Lopez R, Sanaka
MR. Changing trends in age, gender, racial
distribution and inpatient burden of achalasia.
Gastroenterology Res. 2017
Apr;10(2):70-77.
- Fisichella PM, Raz D, Palazzo F, Niponmick I,
Patti MG. Clinical, radiological, and manometric
profile in 145 patients with untreated
achalasia. World J Surg. 2008
Sep;32(9):1974-9.
- Laurino-Neto RM, Herbella F, Schlottmann F,
Patti M. Evaluation of esophageal achalasia:
from symptoms to the Chicago classification. Arq
Bras Cir Dig. 2018;31(2): e1376.
- Torresan F, Ioannou A, Azzaroli F, Bazzoli F.
Treatment of achalasia in the era of
high-resolution manometry. Ann
Gastroenterol. 2015
Jul-Sep;28(3):301-308.
- Eckardt AJ, Eckardt VF. Current clinical
approach to achalasia. World J
Gastroenterol. 2009 Aug
28;15(32):3969-75.
- Genc B, Solak A, Solak I, Serkan Gur M. A rare
manifestation of achalasia: huge esophagus
causing tracheal compression and progressive
dyspnea. Eurasian J Med. 2014
Feb;46(1):57-60.
- Gyawali CP. Achalasia: new perspectives on an
old disease. Neurogastroenterol Motil. 2016
Jan;28(1):4-11.
|



















