|
Introduction
Leptospirosis
is an emerging zoonotic disease caused by
pathogenic species of Leptospira. It is
transmitted to humans primarily through direct or
indirect contact with the urine of infected
animals or contaminated water and soil (1).
Patient presents to the hospital with a broad
spectrum of clinical manifestations, ranging from
a mild, self-limiting febrile illness to severe,
potentially fatal complications involving multiple
organs (2, 3). In many endemic regions,
leptospirosis still remains a leading cause of
pyrexia of unknown origin (PUO) (4). Globally, it
is estimated that leptospirosis accounts for
approximately 1.03 million cases and 58,900 deaths
annually, with the highest burden observed in
tropical and subtropical countries (5). In India,
the disease is particularly prevalent in the
western and coastal states including Gujarat,
Maharashtra, Karnataka, Tamil Nadu, and Kerala
(6).
The incidence
typically peaks during the monsoon and
post-monsoon seasons (June to October), coinciding
with heavy rainfall, flooding, and increased
exposure to contaminated environments (7, 8).
Pathogenic Leptospira penetrate the host
through abraded skin or mucous membranes,
particularly during exposure to contaminated
floodwaters, moist soil, or animal reservoirs such
as rodents, dogs, and cattle [9-11]. Risk factors
include walking barefoot, presence of rodents in
the household, occupational exposure in
agriculture or sanitation, and recreational water
activities (12). It is often a self-limited
disease, with few cases requiring hospitalization.
Although leptospirosis can often resolve without
hospitalization, severe cases—particularly the
icteric form, also known as Weil’s disease can
lead to jaundice, renal failure, hemorrhagic
manifestations, and even death (13). Identifying
local environmental and behavioral risk factors is
crucial for early diagnosis, community education,
and implementing preventive public health
strategies. In this context, the present study
aims to identify environmental risk factors
associated with leptospirosis among hospitalized
patients in coastal Karnataka using a case-control
approach
Materials and Methods
Study Design and Setting
A hospital-based
case-control study was conducted to identify
environmental and behavioral risk factors
associated with leptospirosis. The study was
approved by the Institutional Ethics Committee,
and written informed consent was obtained from all
participants. Data collection was conducted
between June and October 2022, coinciding with the
monsoon season in coastal Karnataka, India, a
region with a known seasonal burden of
leptospirosis.
Study
Participants: Patients presenting with
pyrexia of unknown origin (PUO) were screened.
Blood samples received in the microbiology
laboratory were tested for anti-Leptospira IgM
antibodies using enzyme-linked immunosorbent assay
(ELISA). Based on serology results, participants
were classified as:
i) Cases (n = 42): PUO patients
who tested positive for anti-Leptospira IgM
antibodies and had a history of environmental
exposure (e.g., contact with floodwater, livestock
or pets, rodents, or contaminated soil).
ii) Controls (n = 42): PUO
patients who tested negative for anti-Leptospira
IgM but had a comparable history of exposure.
Inclusion Criteria: Patients
were included if they presented with PUO and if
they were tested for anti-Leptospira IgM by ELISA.
Exclusion Criteria: Patients
were excluded if they were not tested for
anti-Leptospira IgM ELISA.
Data
Collection and Analysis: Data were
collected through a pre-validated structured
questionnaire, which captured information on
demographics, occupational and environmental
exposure, clinical symptoms, prior antibiotic use,
and sanitation practices. Data entry and
management were carried out using Epicollect5
software. Descriptive and inferential statistical
analyses were performed to determine the
association between exposure variables and
leptospirosis.
Results
A total of 84
participants were enrolled in the study,
comprising 42 cases and 42 controls. The
demographic characteristics, clinical history,
prior treatment, and environmental exposure data
were collected using the Epicollect5 platform.
There was no significant difference between cases
and controls in terms of gender distribution
(Figure 1). A substantial proportion of
leptospirosis cases (45.2%) were outdoor unskilled
workers, suggesting a potential occupational
exposure risk. Most cases hailed from semi-urban
(19%) and rural (14.3%) areas. As the study was
conducted during the monsoon season in coastal
Karnataka, nearly all participants, including
controls, reported exposure to rainwater.
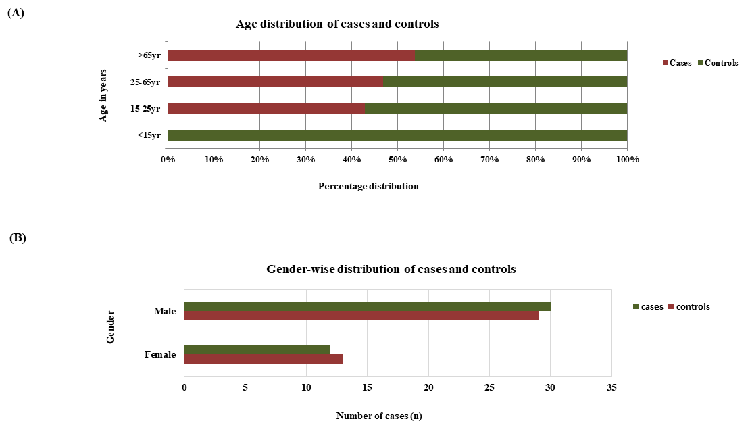
|
| Figure
1: (A) Age Distribution of Cases and
Controls (B) Gender distribution of cases
and controls |
All participants
presented with fever, as per the inclusion
criteria. Prior use of over-the-counter (OTC)
medications before hospital presentation was
reported by approximately 21–23% of both groups.
Among the leptospirosis cases, high-grade fever
was the most common symptom (80.95%), followed by
chills (59.5%), abdominal pain (50%), vomiting
(47%), and jaundice (33.3%). Notably, 95.2% of the
cases had initiated antibiotic treatment prior to
hospital admission, with doxycycline being the
most commonly used drug, which is consistent with
recommended first-line therapy for leptospirosis.
The mean duration of hospital stays for confirmed
cases was 7.52 days, and the majority of these
patients had no previous history of leptospirosis
(Table 1).
|
Table 1: Comparison of
demographic, occupational, clinical, and
treatment characteristics between
leptospirosis cases and controls (n = 42
each)
|
|
Variables
|
Cases (n=42)
|
Controls (n=42)
|
Odds Ratio
|
P value
|
|
Demographic details
|
|
|
|
|
|
Male (n, %)
|
30, 71.4
|
29, 69
|
1.12
|
1
|
|
Occupation (n, %)
|
|
|
|
|
|
Indoor work
|
14, 33.3
|
29, 69
|
0.22
|
0.0022
|
|
Outdoor unskilled work
|
19, 45.2
|
10, 23.8
|
2.64
|
0.0664
|
|
Outdoor semiskilled work
|
8, 19
|
2, 4.7
|
4.71
|
0.0882
|
|
Outdoor skilled work
|
1, 2
|
1, 2
|
1
|
1
|
|
Habitat (n, %)
|
|
|
|
|
|
Rural
|
6, 14.28
|
4, 9.5
|
1.58
|
0.7379
|
|
Semi-urban
|
8, 19
|
1, 2
|
9.65
|
0.0294
|
|
Urban
|
5, 11.9
|
10, 23.8
|
0.43
|
0.2545
|
|
Symptoms (n, %)
|
|
|
|
|
|
Abdominal pain
|
21, 50
|
13, 30.95
|
2.23
|
0.1197
|
|
Vomiting
|
20, 47.61
|
14, 33.33
|
1.82
|
0.2664
|
|
Headache
|
18, 42.85
|
13, 30.95
|
1.67
|
0.3658
|
|
Jaundice
|
14, 33.33
|
3, 7.1
|
6.5
|
0.0055
|
|
High fever
|
34, 80.95
|
28, 66.66
|
2.12
|
0.2147
|
|
Muscle aches
|
22, 52.3
|
19, 45.23
|
1.33
|
0.6624
|
|
Red eyes
|
3, 7.1
|
5, 11.9
|
0.57
|
0.7126
|
|
Diarrhea
|
3, 7.1
|
5, 11.9
|
0.57
|
0.7126
|
|
Rash
|
2, 4.7
|
3, 7.1
|
0.65
|
1
|
|
Chills
|
25, 59.5
|
20, 47.61
|
1.62
|
0.3815
|
|
Other
|
3, 7.1
|
5, 11.9
|
|
|
|
Complications
|
6, 14.28
|
3, 7.1
|
2.17
|
0.4827
|
|
OTC medication use
|
10, 23.8
|
9, 21.4
|
|
|
|
Antibiotic use
|
40, 95.2
|
23, 54.7
|
16.52
|
<0.0001
|
|
Hospitalization
|
41, 97.6
|
38, 90.5
|
4.32
|
0.3597
|
|
Duration of illness (in days)
|
7.52
|
14.36
|
|
|
Animal exposure was
reported by 69% of cases, with an estimated
4.4-fold increased risk of disease compared to
controls. The most frequently reported animals
included dogs, cats, and cattle. Among those
exposed, 33.3% of the cases had open wounds during
their contact with animals or rainwater. These
wounds were most commonly located on the feet,
legs, and fingers. A large proportion (95%) of
cases reported wearing open footwear, increasing
their risk of exposure. Furthermore, 30.95% of
households of the cases lacked proper drainage,
with open drains being reported in their vicinity.
Only 14.3% of cases
reported travel in the two weeks preceding
illness. About 47.6% of the cases gave a history
of animal contact at work, and 26.2% of them had
recent cuts or grazes on their limbs during
exposure. A total of 80.95% of the cases reported
exposure to stagnant water. Among them, 45.2% had
contact with waterlogged areas, 42.9% with wet
soil, 23.8% with floodwater, 11.9% with sewage
water, and 7.1% with standing fresh water or
public pools. Additionally, 35.7% of cases
reported the presence of open sewage around their
homes.
Although the
majority of both cases and controls consumed
treated water, nearly 50% relied on well water, a
common source of drinking water in the coastal
Karnataka region. The use of indoor latrines was
reported by 97.6% of participants, with outdoor
defecation being rare. Public toilet usage was
reported by 14.3%, and 7.1% of patients used other
shared facilities. Importantly, none of the cases
reported wearing protective clothing, and 95%
admitted to not wearing protective footwear while
at work, underscoring significant gaps in personal
protective practices (Table 2).
|
Table 2: Environmental Risk
Factors Associated with Leptospirosis
among Cases and Controls
|
|
Variable
|
Cases (n, %)
|
Controls (n, %)
|
Odds Ratio
|
P-value
|
|
H/o Direct or Indirect Contact
with Animals
|
29, 69
|
14, 33.33
|
4.46
|
0.0021
|
|
Rodents
|
1, 2.4
|
1, 2.4
|
|
|
|
Dogs
|
20, 47.6
|
11, 26.2
|
|
|
|
Cats
|
16, 38.1
|
6, 14.3
|
|
|
|
Cattle
|
14, 33.3
|
3, 7.1
|
|
|
|
Goats
|
3, 7.1
|
1, 2.4
|
|
|
|
Pigs
|
4, 9.5
|
1, 2.4
|
|
|
|
Hens
|
4, 9.5
|
0, 0
|
|
|
|
Cuts/Abrasions at Time of Contact
|
14, 33.3
|
8, 19.0
|
2.12
|
0.2142
|
|
Type of footwear
|
|
|
|
|
|
Closed (Shoes/Boots)
|
2, 4.8
|
5, 11.9
|
0.37
|
0.4326
|
|
Open (Sandals/Chappals)
|
40, 95.2
|
37, 88.1
|
2.7
|
0.4326
|
|
Drainage facilities
|
|
|
|
|
|
Disposed in Environment
|
13, 31.0
|
10, 23.8
|
1.43
|
0.6252
|
|
Municipal
|
17, 40.5
|
26, 61.9
|
0.42
|
0.0802
|
|
Septic Tank
|
12, 28.6
|
6, 14.3
|
2.4
|
0.1828
|
|
Garbage Disposal
|
|
|
|
|
|
Compost Pit
|
11, 26.2
|
8, 19.0
|
1.51
|
0.6028
|
|
Local Area
|
12, 28.6
|
5, 11.9
|
2.96
|
0.1015
|
|
Municipal
|
19, 45.2
|
29, 69.0
|
0.37
|
0.0465
|
|
H/o Travel
|
6, 14.3
|
3, 7.1
|
2.17
|
0.4827
|
|
Animal Contact at Work
|
20, 47.6
|
8, 19.0
|
3.86
|
0.0102
|
|
Recent Cuts/Grazes on Limbs
|
11, 26.2
|
7, 16.7
|
1.77
|
0.4257
|
|
Contact with Stagnant Water
|
34, 81.0
|
27, 64.3
|
2.36
|
0.1412
|
|
Standing Freshwater
|
3, 7.1
|
5, 11.9
|
|
|
|
Floodwater
|
10, 23.8
|
6, 14.3
|
|
|
|
Waterlogged Areas
|
19, 45.2
|
12, 28.6
|
|
|
|
Public Pool
|
3, 7.1
|
0, 0
|
|
|
|
Flowing River
|
2, 4.8
|
1, 2.4
|
|
|
|
Wet Soil
|
18, 42.9
|
14, 33.3
|
|
|
|
Sewage
|
5, 11.9
|
2, 4.8
|
|
|
|
Open Sewage/Trash Near House
|
15, 35.7
|
8, 19.0
|
|
|
|
Consumption of Untreated Water
|
3, 7.1
|
3, 7.1
|
|
|
|
Water Source - River/Lake
|
1, 2.4
|
1, 2.4
|
|
|
|
Water Source - Tap
|
14, 33.3
|
15, 35.7
|
|
|
|
Water Source - Well
|
21, 50.0
|
21, 50.0
|
|
|
|
Water Source - Aquaguard
|
0, 0
|
1, 2.4
|
|
|
|
Water Source - Borewell
|
10, 23.8
|
6, 14.3
|
|
|
|
Sanitary practices
|
|
|
|
|
|
Indoor Latrine
|
41, 97.6
|
41, 97.6
|
1
|
1
|
|
Outdoor Latrine
|
1, 2.4
|
1, 2.4
|
1
|
1
|
|
Nearby Outdoor Latrine
|
2, 4.8
|
1, 2.4
|
2.05
|
1
|
|
Bath at Home
|
33, 78.6
|
40, 95.2
|
|
|
|
Bath at Public Toilet
|
6, 14.3
|
0, 0
|
|
|
|
Bath in Public Area
|
3, 7.1
|
2, 4.8
|
|
|
|
Personal Habits
|
|
|
|
|
|
Protective Footwear at Work
|
2, 4.8
|
6, 14.3
|
0.3
|
0.2646
|
|
Protective Clothing at Work
|
0, 0
|
2, 4.8
|
0
|
0.494
|
|
Hand Hygiene Before Breaks
|
30, 71.4
|
39, 92.9
|
0.19
|
0.0204
|
|
Recall Bias
|
|
|
|
|
|
Recall - Not Well
|
1, 2.4
|
10, 23.8
|
|
|
|
Recall - Very Well
|
4, 9.5
|
4, 9.5
|
|
|
|
Recall - Well
|
37, 88.1
|
28, 66.7
|
|
|
Discussion
In India the west
coastal states of Gujarat, Maharashtra, Goa,
Karnataka and Kerala are affected by
leptospirosis. The positivity rate for the disease
is notable in the southern part of India at 25.6%,
followed by 8.3%, 3.5%, 3.1%, and 3.3% in
northern, western, eastern and central India,
respectively (14). Agricultural activities,
contact with farm animals, exposure to sewage
water are most common modes of exposures for
acquiring leptospirosis in India (15). There is no
gender difference seen among cases verses controls
in our study. Majority (45.2%) cases were outdoor
unskilled workers. Heavy rain falls, improper
drainage system, outdoor activities which exposed
them to mud, stagnant water and animals(P=0.001),
not wearing footwear or wearing open foot wear
were significantly associated with leptospirosis
cases in our study which is concordant with other
studies conducted in India (16, 17). Exposure to
waterlogged areas is seen in 45.23% of cases which
is significantly higher in infected cases. These
waterlogged areas act like reservoirs where the Leptospira
multiply and present in high number.
History of direct
exposure to rodents is less in our study compared
to other animals like cattle, dogs, cats which is
contrast to other studies conducted in India (17,
18). But in any farming activity, there will be
wastages which will attract the rodents resulting
in mixing of rodent urine in the soil and water.
Patients can get exposed to such soil or water
with Leptospira unknowingly and get the
disease. There was a history of cut in the skin in
33.3% cases compared to 19.04% of controls (Figure
2). A study by Udayar et al. found significant
association between skin wounds and leptospirosis
infection (17). 95% patients gave the history of
not wearing protective clothing or footwear while
doing outdoor activities. These practices along
with non-intact skin will make them prone for
acquiring the disease.
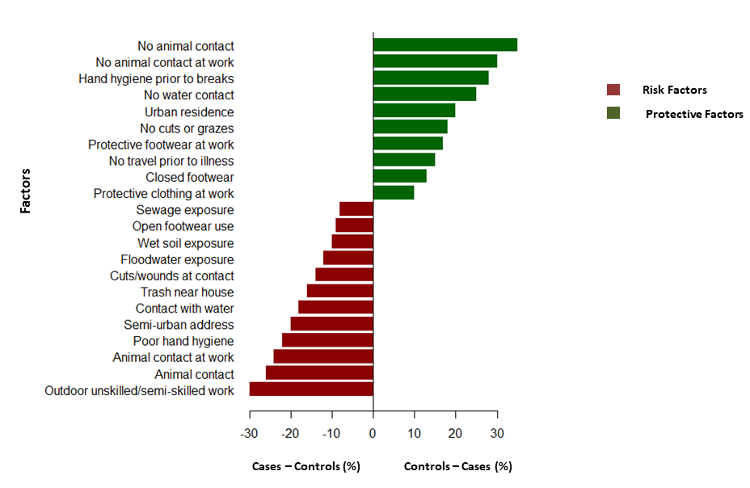
|
| Figure
2: Graphical abstract on good and bad
practices in leptospirosis along with
percentage predisposition to disease due
to the features |
In developed
countries, the disease is most often recognized in
people with occupational activities that involve
water exposure or interactions with animal
reservoir hosts or in people participating in
recreational activities involving water. Wildlife
trapping for research purposes, production animal
work (abattoir work, dairy farming, veterinarians
working with livestock), water-intensive crop
farming (bananas, pineapples, taro, rice,
berries), military operations, fish farming, and
sewer work increase risk for leptospirosis (18,
19).
In India the
majority of leptospirosis cases are found in the
western and coastal states. Most of the patients
(74.7%) recover without any complications and
nearly one-fifth of them recovered with
complications (21.8%). Acute renal failure was the
most commonly seen (79.2%) complications. The case
fatality was found to be 3.5%. There was a
significant increase in the mortality documented
from the state of Kerala when the infected
patients have other comorbid conditions or when
they have infection due to other hemorrhagic
viruses. As the environmental factors responsible
for Leptospirosis and hemorrhagic fevers like
dengue are similar, there was significant
co-infection found to as high as 17.5% from
southern part of India (20).
Our study shows the
importance of basic behavioral changes required
for the prevention of Leptospira infection
and its complications. Measures like protective
clothing, footwear, gloves while working in paddy
field and farms to avoid direct contact with the
contaminated soil or stagnant water are essential
to avoid any infection. There is a need of
awareness in the people and health education
should be given regarding disease and its risk
factors.
Conclusion
This study
underscores the significant association between
environmental exposures and the occurrence of
leptospirosis among hospitalized patients in
coastal Karnataka. Factors such as direct or
indirect contact with animals, exposure to
stagnant or contaminated water, lack of protective
footwear and clothing, and presence of open wounds
were strongly associated with increased risk of
infection. The findings highlight the need for
targeted public health measures, including
community awareness, personal protective
practices, and improved sanitation infrastructure,
particularly during the monsoon season.
Identifying and addressing these risk factors is
essential for early diagnosis, prevention, and
reduction in disease burden in endemic regions.
Conflict of Interest:
Authors declare no conflict of
interest
Acknowledgement: We would like to thank the
technical staff at the department of
Microbiology for helping in performing IgM ELISA
Funding: No
funding was received for this study
References
- Thayaparan S, Robertson ID, Fairuz A, Suut L,
Abdullah MT. Leptospirosis, an emerging zoonotic
disease in Malaysia. Malays J Pathol. 2013
Dec;35(2):123-32
- Rajapakse S. Leptospirosis: clinical aspects.
Clin Med (Lond). 2022 Jan;22(1):14-17
- Samrot AV, Sean TC, Bhavya KS. Leptospiral
Infection, Pathogenesis and Its Diagnosis-A
Review. Pathogens. 2021 Feb 1;10(2):145
- James S, Sathian B, van Teijlingen E, Asim M.
Outbreak of Leptospirosis in Kerala. Nepal J
Epidemiol. 2018 Dec 31;8(4):745-747
- Costa F, Hagan JE, Calcagno J. Global
Morbidity and Mortality of Leptospirosis: A
Systematic Review. PLoS Negl Trop Dis.
2015 Sep 17;9(9): e0003898
- Gupta N, Wilson W, Ravindra P. Leptospirosis
in India: a systematic review and meta-analysis
of clinical profile, treatment and outcomes. Infez
Med. 2023 Sep 1;31(3):290-305
- Manjunathachar HV, Barde PV, Chouksey V.
Leptospirosis in central India: A retrospective
study to explore burden of tropical illness. Indian
J Med Microbiol. 2024 Sep-Oct; 51:100689
- Pawar S, Kore M, Athalye A, Thombre PS.
Seasonality of leptospirosis and its association
with rainfall and humidity in Ratnagiri,
Maharashtra. Int J Health Allied Sci. 2018
Jan 1;7(1):37-40
- Sykes JE, Haake DA, Gamage CD. A global one
health perspective on leptospirosis in humans
and animals. J Am Vet Med Assoc. 2022
Jul 25;260(13):1589-1596
- Mwachui MA, Crump L, Hartskeerl R.
Environmental and Behavioural Determinants of
Leptospirosis Transmission: A Systematic Review.
PLoS Negl Trop Dis. 2015 Sep 17;9(9):
e0003843
- Bhardwaj P, Kosambiya JK, Desai VK. A case
control study to explore the risk factors for
acquisition of leptospirosis in Surat city,
after flood. Indian J Med Sci. 2008
Nov;62(11):431-8
- Haake DA, Levett PN. Leptospirosis in humans.
Curr Top Microbiol Immunol. 2015;
387:65-97
- Sellors P, Watson RF, Bate R. Clinical
Features and Severity of Leptospirosis Cases
Reported in the Hawke's Bay Region of New
Zealand. J Trop Med. 2021 Jul 6;
2021:5567081
- DHS. Epidemiological Situation of Communicable
Diseases in Kerala (2006-2010). Integrated
disease surveillance project. Available from: http://dhs.kerala.gov.in/docs/part1.pdf
- Antima, Banerjee S. Modeling the dynamics of
leptospirosis in India. Sci Rep. 2023
Nov 13;13(1):19791
- Patil DY, Dahake RV, Chowdhary AS.
Clinico-epidemiological observations of human
leptospirosis from Mumbai, India. J Infect
Public Health. 2017 Mar-Apr;10(2):247-248
- Udayar SE, Chengalarayappa NB, Madeshan A.
Clinico Epidemiological Study of Human
Leptospirosis in Hilly Area of South India-A
Population Based Case Control Study. Indian
J Community Med. 2023
Mar-Apr;48(2):316-320
- Kembhavi RS, Velhal GD, Shah AK.
Epidemiological determinants of leptospirosis in
rural and urban districts of Maharashtra, India.
J Family Med Prim Care. 2021
Sep;10(9):3361-3367
- Monahan AM, Miller IS, Nally JE.
Leptospirosis: risks during recreational
activities. J Appl Microbiol. 2009
Sep;107(3):707-16
- Sachu A, Madhavan A, Vasudevan A. Prevalence
of dengue and leptospirosis co-infection in a
tertiary care hospital in south India. Iran
J Microbiol. 2018 Aug;10(4):227-232.
|



















