|
Introduction
Breast
cancer has been ranked as number one cancer among
Indian females with a mortality of 12.7 per
100,000 women and age adjusted rate as
high as 25.8 per 100,000 women. [1] Five year
survival rate after primary diagnosis is about 80-
90 %, even though 4.5 million succumb annually to
breast cancer.[2] Prognosis in breast cancer
determines the survival. Lymph node metastasis,
lymphovascular invasion, oestrogen receptor alpha,
progesterone receptor, proliferation rate,
Her2Neu, size of the tumour, histological grade,
intrinsic subtypes and tumour buds (both intra and
inter tumoral buds) are important established
prognostic factors in breast cancer. One of the
new prognostic factor emerging in breast cancer
prognosis is adipose tissue invasion in the tumour
margin.
Breast tissue is
composed by 90% of adipose tissue (AT) with
permanent interactions between epithelial cells
and adipose cells. [3] AT is a loose connective
tissue characterized by marked cellular
heterogeneity. It is made up of about one-third of
adipocytes and two-thirds of stromal-vascular
fraction cells, a combination of mesenchymal stem
cells, endothelial precursor cells, fibroblasts,
smooth muscle cells, pericytes, macrophages and
preadipocytes in various stages of development.
[4] In the mammary gland, adipose cells are
characterized by high plasticity and support the
growth and function of the mammary epithelium. [5]
Adipose cells communicate with cancer cells within
the breast, and this may contribute to cancer
progression, through different mechanisms of
mechanical support and energy supply.
Invasion of tumour
cells into the marginal adipose tissue (ATI) leads
to larger area of contact between peritumoral
lymphatics and tumour cells. Functional lymphatics
at the peritumoral site is mainly responsible for
lymphovascular invasion. Once tumour cells come in
contact with peritumoral lymphatics, it leads to
increased chances of lymphovascular invasion (LVI)
which is a bad prognostic indicator ultimately
leading to lymph node metastasis (LNM).
Few authors had
previously studied the association of adipose
tissue invasion by tumour cells with other clinic-
histological prognostic indicators. [6-8] The
present study was conducted with the aim to
evaluate whether ATI of cancer cells at the tumour
margin influenced lymph node status and prognosis
in patients with invasive ductal carcinoma of the
breast.
Materials and Methods:
This was a
cross-sectional study conducted over a duration of
three years from January 2019 up to December 2022.
Ethical approval for this study (IEC/AIMS-13/2018)
was provided by institutional ethical committee,
AIMS, on 12th December 2018.
Study
population and demographics:
Study
populationincluded43 cases of invasive ductal
carcinoma diagnosed at histopathology in the
department of Pathology during the study period.
Written informed consent from the patients was
obtained. Convenient sampling technique was
followed. Cases with special histologic type such
as mucinous, lobular, medullary, or squamous cell
carcinoma were excluded from the study. Patients
with bilateral breast cancer, multifocal or
multicentric tumours in the unilateral breast,
skin or striated muscle invasion, inflammatory
carcinoma, distant metastasis, or malignancy at
another site and for patients who had received
preoperative neoadjuvant chemotherapy were also
excluded from the study.
Study population was
divided in two groups, one with adipocyte tissue
invasion (ATI +) which included 33 cases and the
other group without adipocyte tissue invasion
(ATI-) comprising of 10 cases.
Processing
of samples:
The resected breast
lesions or core biopsy specimens were fixed using
10% neutral buffered formalin. Representative bits
were processed by routine paraffin embedding.
Subsequently, 4 micron thick sections were
prepared and stained with haematoxylin and eosin
stain. Lymph nodes were extracted from modified
radical mastectomy specimens and examined
histopathologic ally for presence of absence of
metastasis.
Definition
of variables:
Adipocyte
tissue:
Adipose tissue was
defined as a pure aggregate consisting of more
than 20 fat cells without intervening fibrous
tissue in the breast. The adipose tissue included
tissue surrounding the mammary ducts or lobules
and those in the subcutaneous layers.
Marginal
adipocyte tissue invasion:
Marginal ATI was
defined as the presence of more than 20 cancer
cells in direct contact with the adipose tissue or
the location of cancer cells in the adipose
tissue.[6] Only cases with unequivocal
ATI were considered positive (ATI+) and doubtful
cases were considered negative (ATI–).
Lymphatic
vessel invasion:
Lymphatic vessel
invasion was defined as the presence of neoplastic
emboli within the endothelium-lined spaces in
areas adjacent to but outside the margins of the
carcinoma. [8] Modified Bloom Richardson
histologic grading system was used for grading of
the tumours at histopathology.
Tumour size:
Tumour size was
measured as the largest dimension of the
microscopic invasive component on the pathologic
specimen and lymph node involvement was recorded
histologically.
Clinicopathologic
prognostic parameters of 33 cases of invasive
ductal carcinoma with marginal ATI were compared
with that of 10 cases without ATI. Value of the
combination of ATI and peritumoral lymphatic
vessel invasion (LVI) was also assessed.
Statistics:
Statistical analyses
were performed using IBM Statistical Package for
the Social sciences (SPSS) Statistics for Windows,
version 24.0 (IBM Corp., Armonk, NY) and the
results were expressed as mean, standard deviation
and percentage. Chi square test was applied for
comparison of categorical variables in the two
groups. The statistical significance was set at
p<0.05.
Results:
A total of 43 cases of infiltrating ductal
carcinoma in whom modified radical mastectomy was
performed were considered for the study.
Demographic
details: (Table 1)
|
Table 1: Shows demographic
profile among cases with and without
adipocyte tissue invasion
|
|
Demographic variables
|
ATI present
|
ATI Absent
|
|
Age (Mean, SD)
|
43± 0.1 years
|
55+1 years
|
|
Laterality
|
|
Right sided
|
10
|
14
|
|
Left sided
|
10
|
09
|
|
Focality
|
|
Unifocal
|
20
|
23
|
|
Multifocal
|
0
|
0
|
|
Nipple discharge
|
4
|
2
|
|
Nipple retraction
|
1
|
2
|
|
Clinical size in cm (Mean, SD)
|
4.3 ± 2 cm
|
3.4
|
|
*SD-Standard deviation
|
Out of 43 cases 14
(32.5%) cases were in the age group of 41 to 50
years, followed by 13 (30.2 %) cases in 51 to 60
years age group. Majority of the cases were seen
in the right side of the breast, 24/43, (55.8%)
and all were unifocal and unilateral tumours.
Maximum number of the cases 34/43 (79%) presented
with lump in the breast, followed by lump in the
breast with nipple discharge 6/43 (13.4%) and lump
in the breast with nipple retraction 3/43 (6.9%).
Quadrant wise distribution of the cases showed
maximum number of cases 11/43 (25.83%) in the
upper outer quadrant followed by lower outer
quadrant 10/43 (22.08%).
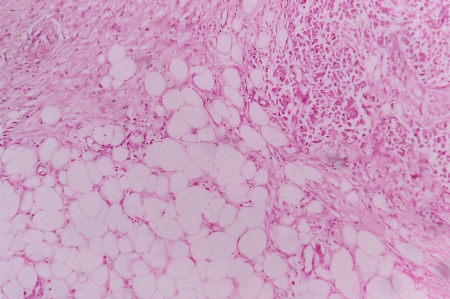
|

|
| Figure
1: Section shows marginal adipose tissue
which was defined as a pure aggregate
consisting of more than 20 fat cells
without intervening fibrous tissues in the
breast. (Haematoxylin and Eosin, x 400)
|
Figure
2: Section shows marginal adipose tissue
invasion which was defined as the presence
of more than 20 cancer cells in direct
contact with the adipose tissue or the
location of cancer cells in the adipose
tissue. (Haematoxylin and Eosin, x 400)
|
Among total 43 cases
of infiltrating ductal carcinoma examined,
adipocyte tissue invasion was noted in 33
(76.74%). Table 1 shows demographic profile of
cases with and without adipocyte tissue invasion.
Association of Adipose tissue invasion
with various clinicopathologic prognostic
parameters: (Table 2)
|
Table 2: Association of adipocyte
tissue invasion (ATI) with various
pathologic prognostic parameters in 33
cases of invasive ductal carcinoma of
the breast
|
|
Parameters
|
ATI present (n-33)
|
ATI absent (n-10)
|
p-value
|
|
Lymph node metastasis
|
|
Present
|
20
|
0
|
<0.001*
|
|
Absent
|
13
|
10
|
|
Lymphatic vessel invasion
|
|
Positive
|
24
|
3
|
0.014*
|
|
negative
|
9
|
7
|
|
Histological grade
|
|
1
|
9
|
6
|
0.162
|
|
2
|
11
|
2
|
|
3
|
12
|
2
|
|
Perineural invasion
|
|
Positive
|
3
|
2
|
0.375
|
|
Negative
|
30
|
8
|
|
Surgical margin invasion
|
|
Positive
|
8
|
0
|
0.165
|
|
Negative
|
25
|
10
|
|
ATI-Adipocyte tissue invasion
Statistical test applied-Chi square test,
statistical significance set at p<0.05,
*- Statistically significant
|
The frequency of
axillary lymph node metastases was higher in
patients with ATI (20/33, 63.7%) as compared to
that without ATI (0/10,0%) and this difference was
found to be statistically significant (p <
.001). The frequency of peritumoral lymph vascular
invasion was higher in patients with ATI (24/27,
88.88%) as compared to that without ATI (3/27,
11.1%) and this difference was also found to be
statistically significant (p < .001). In
addition, patients without ATI or LVI had no lymph
node metastasis (n =-7).
Mean tumour size was
significantly higher ATI+ cases as compared to
that without ATI ((2.2 +0.3 cm vs 1.1+0.1 cm,
p<0.001)
Among total 33 cases
with ATI, maximum number of cases were
histopathologically grade 3 tumours (13/33,39.3%),
followed by grade 2 (11/33,33.3%) and grade 1
(9/33,27.2%) tumours. In contrast to this, among
total 10 cases without ATI, maximum cases
(6/10,60%) were grade 1, whereas two cases each
(20%) were grade 2 and 3. Surgical margin invasion
was seen in only 8/33 (24.2%) cases of ATI, whilst
none of the cases without ATI showed surgical
margin invasion. Perineural invasion was present
in 9.09% (3/33) of cases with ATI and 20% (2/10)
of cases without ATI. There was no statistically
significant association between presence of
absence of ATI and tumour grade, surgical margin
invasion and perineural invasion (p- 0.162,
p-0.014 and p-0.375) respectively.
Discussion:
Adipocyte tissue
next to breast cancer cells show modulation of
gene expression profile in the form of
down-regulation of the adipogenesis-related genes
Homeobox C Cluster (HOXC)8, HOXC9, fatty acid
binding protein 4 (FABP4) and hormone sensitive
lipase (HSL) and up-regulation of inflammatory
cytokines, like TNF-α, monocyte chemoattractant
protein 1 (MCP-1), leptin, with a decrease of
adiponectin levels.[9] Compared to normal
adipocytes, the so called “cancer- associated
adipocytes” (CAA) are smaller cells, with a
reduction in the number and size of lipid droplets
and modification of basement membrane proteins.
[10] The reduction of lipid droplets takes place
with the metabolic reprogramming that adipocytes
undergo in contact with breast cancer cells and
with the acquisition of a brown-fat like
phenotype. Indeed, cancer cells induce the
lipolysis in CAAs via HSL and adipose triglyceride
lipase (ATGL). [10] Despite lower adiposity, Xbp1s
overexpression in these cells promote tumour
progression.[11] These modifications in the AT
close to tumour cells are responsible for
promoting tumour progression and influencing the
biologic behaviour of the breast cancer. This
adipocyte tumour cell cycle may be a potential
target for therapeutic interventions in future.
Earlier studies have
envisaged the significance of dispersed adipocyte
invasion and invasive length of adipose tissue
invasion as a marker of prognosis in breast
cancer. Very few studies have evaluated the role
of ATI at the tumour margin in prognostication in
breast cancer.
The present study
showed significant positive association of ATI
with lymphovascular invasion. This was in
concordance with the findings reported by Moriuchi
H et al.[7] Though no prognostic significance was
noted between ATI + cases and LVI +, ATI - and
LVI-cases did not show evidence of any recurrence
or metastasis among the study population reported
by Yamaguchi et al.[6] This finding further
portrays combined utility of ATI and LVI in
determining the need for axillary dissection or
chemoradiotherapy post-surgery. Predictive value
of peritumoral LVI in lymph node metastasis has
been well established and is considered as an
adverse prognostic indicator. Similarly, tumour
cells in contact with peritumoral lymphatics not
only leads to nodal but also distant metastasis.
Our recent work highlights the utility of D2-40
stain in determining lymphatic vessel density in
invasive breast carcinoma Amita et al reported
significant positive correlation between
intratumoural and peritumoral lymphovascular
density and tumour size and stage in breast cancer
patients. Similarly, higher peritumoral
lymphovascular density was associated with
LVI.[12] Further studies exploring molecular
markers directed towards development for newer
therapeutic targets are essential.
The current study
also showed that the ATI had significant
association with lymph node metastasis. The
frequency of axillary lymph node metastases was
significantly higher in patients with ATI as
compared to that without ATI.
Yamaguchi et al,
studied 735 early invasive breast cancer cases of
both luminal type and triple negative breast
cancer. Authors observed that ATI was strongly
associated with poor survival in triple negative
breast cancer. Their findings recognized the
invasion of cancer cells in adipocytes as the
earliest step in cancer progression.[6] One of the
major limitation of the present study was the
inability to assess the prognostic significance of
ATI and LVI with the molecular subtypes of breast
cancer.
In the present
study, there was no significant association of
tumour size with ATI. Small sample size and less
number of cases with large tumour size, in the
present study, limits the generalization of this
finding. These findings were contrary to that
reported by Yamaguchi et al and Moriuchi et al,
who observed that larger tumours had higher rate
of adipose tissue invasion.[6,7]
In the present
study, tumours with ATI had higher grades, whereas
tumours without ATI showed more cases with grade 1
morphology on histopathology. Nevertheless, grade
of the tumour did not show any significant
association with the ATI. Similar findings were
reported by Moriuchi et al.[7] However in contrast
to this, Yamaguchi et al reported grade 3 tumours
to be associated with adipose tissue invasion.[6]
This indicates that more than aggressiveness of
the tumour cells there may be other factors that
may paly role in the interaction between tumour
cells and adipocytes which needs further
investigation.
Age did not have any
impact on ATI in the present study. Similar
findings have been reported in the literature.
However, with regard to LVI, studies have reported
LVI to associated with younger age group. This
phenomenon has been attributed to lesser fat
content and aggressive nature of cancer cells in
younger patients in contrast to the older
population.
In the present
study, tumour size was significantly higher in
ATI+ cases as compared to ATI- cases. The
significance of tumour size in lymph node
metastasis is a well-established fact.[13] Larger
size tumours have greater opportunity to interact
closely with the adipocytes, thereby paving way
for altering the tumour microenvironment in favour
of intravasation and metastasis.
Adipocyte tumour
invasion (ATI) of cancer cells at the tumour
margin is one of the biologic indicators of tumour
aggressiveness in invasive breast cancer. The
present study showed that ATI had significant
association with well-established prognostic
parameters like nodal metastasis and LVI. The
present study emphasized that ATI was an
independent factor influencing nodal metastasis.
Conclusion:
Presence of
adipocyte tissue invasion was associated with LVI
and lymph node metastasis. ATI and peritumoral LVI
can serve as novel biomarker for prognosis, risk
stratification and planning treatment. However,
further studies involving large sample size must
be conducted for generalization of the results. If
proved as proposed, ATI could be incorporated in
synoptic cancer reporting protocol of infiltrating
ductal carcinoma of the breast.
References:
- Malvia S, Bagadi SA, Dubey US, et al.
Epidemiology of breast cancer in Indian women. Asia
Pac J Clin Oncol. 2017;13:289–95.
- Giaquinto AN, Sung H, Miller KD, et al. Breast
Cancer Statistics, 2022. CA Cancer J Clin.
2022;72:524-41.
- Pallegar NK, Christian SL. Adipocytes in the
tumour microenvironment. Adv Exp Med Biol.
2020;1234:1–13. 10.1007/978-3-030-37184-5_1
- Frühbeck G. Overview of adipose tissue and its
role in obesity and metabolic disorders. Methods
Mol Biol. 2008;456:1–22.
10.1007/978-1-59745-245-8_1
- Hovey RC, Aimo L. Diverse and active roles for
adipocytes during mammary gland growth and
function. J Mammary Gland Biol Neoplasia.
2010;15:279–90.
- Yamaguchi J, Ohtani H, Nakamura K, et al.
Prognostic Impact of Marginal Adipose Tissue
Invasion in Ductal Carcinoma of the Breast. American
journal of Clinical Pathology.
2008;1382–8.
- Moriuchi H, Yamaguchi J, Hayashi H, et al.
Cancer Cell Interaction with Adipose Tissue:
Correlation with the Finding of Spiculation at
Mammography. Radiology. 2016;279:56–64.
- de Mascarel I, Bonichon F, Durand M, et al.
Obvious peritumoral emboli: an elusive
prognostic factor reappraised. Multivariate
analysis of 1320 node-negative breast cancers. Eur
J Cancer. 1998;34:58–65.
- Wang F, Gao S, Chen F, et al. Mammary fat of
breast cancer: gene expression profiling and
functional characterization. PLoS One.
2014;9:e109742.
- Choi J, Cha YJ, Koo JS. Adipocyte biology in
breast cancer: from silent bystander to active
facilitator. Prog Lipid Res.
2018;69:11–20. 10.1016/j.plipres.2017.11.002
- Zhu Q, Zhu Y, Hepler C et al. Adipocyte
mesenchymal transition contributes to mammary
tumor progression. Cell Rep. 2022 Sep
13;40(11):111362. doi:
10.1016/j.celrep.2022.111362.
- Dhanlakshmi B, Amita K, Prashantha K.
Prognostic Significance of Lymphatic Vessel
Density by D2-40 Immune Marker and Mast Cell
Density in Invasive Breast Cancer: A Cross
Sectional Study at Tertiary Care Hospital in
South India. Online J Health Allied Scs. 2022;21:5.
- Seidman JD, Schnaper LA, Aisner SC.
Relationship of the size of the invasive
component of the primary breast carcinoma to
axillary lymph node metastasis. Cancer. 1995;75:65-71.
|



















