|
Introduction
Urinary
tract infections (UTIs) represent widespread human
microbial disorders involving any part of the
urinary tract, such as the kidneys, bladder,
urethra, and prostate .1 UTI infection
exhibits a variety of symptoms including mild
burning micturition, bacteremia, sepsis and even
death.2 It is reported that UTI affects
both genders but women in the age group 15-44 are
more prone to this infection.3 Amongst
the various types of urinary tract infection,
cystitis (lower urinary tract infection) and
pyelonephritis (upper urinary tract infection)
constitute the majority of problems. The most
common symptoms of lower urinary tract infection
include inflammation and irritation in the lining
of urethra and bladder, burning sensation or pain
while urinating. Other symptoms include frequent
urination often with scanty urine, sensation of
having to urinate urgently, cloudy, bad smelling,
or bloody urine, lower abdominal pain and
sometimes mild grade fever. The most frequent
upper urinary tract infections symptoms include
high grade fever, nausea and vomiting, shaking
chills, pain in back or side of waist. In children
when compared with adults, fever, vomiting, loss
of bladder control and sleep are more common
symptoms.
Urinary tract
infections (UTIs) are prevalent all over the world
and have both direct and indirect influences on
the socioeconomic parameters in the global
population. Moreover, these infections contribute
to significant burden of morbidity and mortality
and are placed second only to respiratory tract
infections.4 UTIs are known to affect
approximately 150 million people each year
worldwide.5
UTIs are categorized
as community or hospital-acquired infections based
on the setting where the infection is acquired.
The community- acquired urinary tract infections
are acquired in a community setting or within the
first 48 hours of hospitalization.6 In
community and hospital settings the etiology of
UTIs and the antimicrobial susceptibility of
uropathogens have been changing over the years.7,8
Factors such as the changing patient
population, extensive use and misuse of
anti-microbial agents have contributed to changes
in the bacterial profile of UTI.9
Knowledge of the
antimicrobial resistance patterns of common
uropathogens according to local epidemiology is
essential for providing clinically appropriate,
cost effective therapy for UTI.10,11
This study was
undertaken to assess the bacterial and demographic
profile of human population presenting with
symptoms of urinary tract infections over a period
of one year between June 2021 to July 2022.
Methods
A retrospective
analysis of data from urine bacteriology seat over
a period of one year between June 2021 to July
2022 was done in the department of Microbiology,
University College of Medical sciences and
associated GTB Hospital, Delhi. The urine samples
received for culture and sensitivity were streaked
on McKonkey Agar and blood agar plates. The plates
were incubated for 24 hours at 37°C. Plates
showing growth suggestive of significant
bacteriuria, with colony counts exceeding 105cfu/ml
were subjected to standard biochemical tests for
identification and antimicrobial sensitivity
testing by Kirby-Bauer disc diffusion method. The
bacterial isolates were interpreted as 'Sensitive'
or 'Resistant' on the basis of the diameters of
zones of inhibition of bacterial growth as per
recommendations by the disc manufacturer.
Statistical
analysis
The data collected
was entered into Microsoft EXCEL spreadsheet and
data analysis was done using, Statistical Package
for Social Sciences (SPSS) software‟, IBM
manufacturer, Chicago, USA, version 20.0.
Results
|
Table 1: Gender wise distribution
of cases
|
|
Male (%)
|
Female (%)
|
Total
|
|
974 (43%)
|
1291 (57%)
|
2265
|
In this study, a
total of 21359 samples were received for urine
culture and sensitivity testing. Out of these,
2265 samples were positive with an overall
positivity rate of 10.60%. Table 1 shows gender
wise distribution of cases. It can be seen that
majority of the positive samples were from females
as compared to males (Positivity rate F: M= 6.04%
: 4.56 %; F:M ratio= 1.8:1)
|
Table 2: Age wise distribution of
cases
|
|
Age
|
Male
|
Female
|
Total
|
|
0-10
|
68
|
107
|
175
|
|
11-20
|
93
|
98
|
191
|
|
21-30
|
265
|
348
|
613
|
|
31-40
|
87
|
277
|
364
|
|
41-50
|
169
|
158
|
327
|
|
51-60
|
124
|
131
|
255
|
|
61-70
|
71
|
117
|
188
|
|
71 onwards
|
97
|
55
|
152
|
|
Total
|
974
|
1291
|
2265
|
Table 2 shows age
wise distribution of positive cases. It can be
seen that majority of positive samples (1559;
68.83%) were from age group between 21-60 years of
age. Females formed the major group (914; 58.62%)
as compared to males in this.
|
Table 3: Distribution of isolates
in OPD, IPD and ICU services
|
|
Organism
|
OPD
|
IPD
|
ICU
|
|
Gram Positive Cocci (GPC)
|
111
|
145
|
22
|
|
Gram Negative Bacilli (GNB)
|
775
|
972
|
149
|
|
Mixed Infection (GPC + GNR)
|
58
|
31
|
1
|
|
Total
|
944
|
1148
|
173
|
Table 3 shows
distribution of isolates in OPD, IPD and ICU
services. Overall, Gram negative bacilli were seen
in 1896 samples followed by Gram positive cocci in
278 positive samples and mixed growth was seen in
92 positive samples. In OPD, IPD and ICU services,
Gram negative bacilli were the predominant
microorganisms isolated.
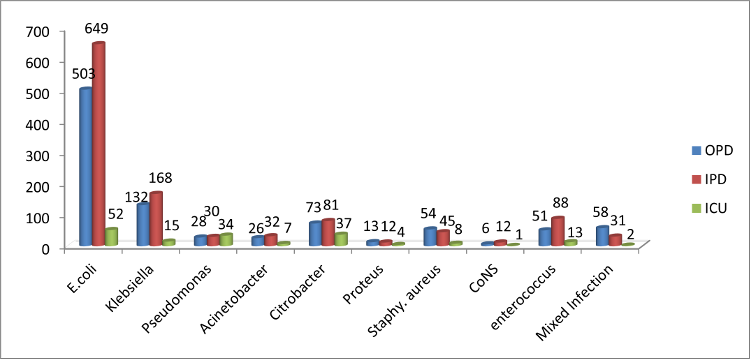
|
| Figure
1: Distribution of isolates in OPD, IPD
and ICU services. |
Figure 1 shows
distribution of isolates in OPD, IPD and ICU
services. In Gram negative bacilli, E. coli
(1204; 53.15%) is the predominant organism
followed by Klebsiella spp. (315;13.90%) and
Citrobacter spp.(191; 8.43%). In Gram positive
cocci, Enterococcus (152;6.71%) was the
predominant species followed by staphylococcus
spp. (107; 4.72%).
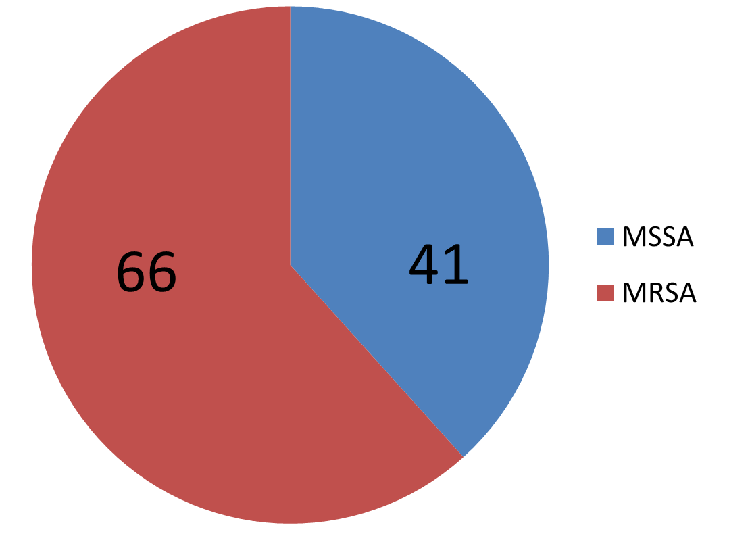
|
| Figure
2: Distribution of Methicillin Resistant
Staphylococcus aureus (MRSA) and
Methicillin Susceptible Staphylococcus
aureus (MSSA) (N=107) |
Figure 2 shows
distribution of Methicillin Resistant Staphylococcus
aureus (MRSA) and Methicillin Susceptible Staphylococcus
aureus (MSSA) among 107 isolates of Staphylococcus
aureus isolated from urine samples between
July 2021 to June 2022.It can be seen that
majority ,i.e., 66 (61.68%)out of total 107 of
isolates were resistant to methicillin as compared
to 41 (38.31%) isolates which were sensitive.
Discussion
In this study, a
total of 21359 samples were received for urine
culture and sensitivity testing. Out of these,
2265 samples were positive with an overall
positivity rate of 10.60%. The majority of the
positive samples were from females as compared to
males (Positivity rate F: M= 6.04%: 4.56 %; F:M
ratio= 1.8:1) proving the fact that there is a
higher preponderance of urinary tract infections
in females as compared to males. The high
incidence of urinary tract infections in females
may be due to shorter urethra in females,
proximity of urethral opening to both the vagina
and the anus, the main source of bacteria such as
Escherichia coli that cause UTIs.12 Our
findings are supported by studies by M.
Muthulakshmi et al and Manjula N.G. et al.2,13
In this study, it
was seen that majority of positive samples (1559;
68.83%) were from age group between 21-60 years of
age. Females formed the major group (914; 58.62%)
as compared to males in this. The incidence of UTI
in females increases with age and sexual activity.14
Post-menopausal women have higher rates of UTIs
because of pelvic prolapse, lack of oestrogen,
loss of lactobacilliin the vaginal
flora, increased periurethral colonization by Escherichia
coli, and a higher incidence of medical
illnesses such as diabetes mellitus (DM).15 Our
findings are well supported by similar study by
Mohapatra et al who reported higher incidence of
UTIs in adult females.16
In this study, 175
(7.72%) out of 2265 total positive samples were
from children between 0-10 years of age. Out of
the total 175 positive samples, 107 (61.14%) were
from girls and 68 (38.85%) were from boys. The
reasons for childhood UTI may be due to
vesicoureteral reflux with pathogenesis of renal
scarring, reflux nephropathy, pyelonephritis and
voiding disorders.17
Similar findings
were observed in study done by Christy VR et al
who reported that out of 79 suspected cases, 28
(35.44%) are culture positive in boys and in girls
it was 37 (46.83%) in the age group 0-10 years.18
In this study, Gram
negative bacilli were seen in 1896 positive
samples followed by Gram positive cocci in 278
positive samples and mixed growth was seen in 92
positive samples from OPD, IPD and ICU services.
The Gram negative bacilli were the predominant
microorganisms isolated from our hospital clinical
services. In Gram negative bacilli, E. coli (1204;
53.15%) is the predominant organism followed by
Klebsiella spp. (315;13.90%) and Citrobacter
spp.(191; 8.43%). In Gram positive cocci,
Enterococcus (152; 6.71%) was the predominant
species followed by staphylococcus spp. (107;
4.72%) [Table 3; Fig.1].Our finds are well
corroborated by studies from Foxman, Nielubowicz
et al, Kline et al and Ronald.19-22
It was seen in our
study, that majority, i.e., 66 (61.68%) out of
total 107 isolates of Staphylococcus aureus
were resistant to methicillin as compared to 41
(38.31%) sensitive isolates. The majority of MRSA
isolates were from OPD followed by IPD and ICU
(37;56.06% >24;36.36% >5;7.57%). The rising
trend of methicillin resistant Staphylococcus
aureus in outpatient department is worrisome as it
represents the load of MRSA in community acquired
urinary tract infection. The rising trend of MRSA
in community acquired urinary tract infection is
attributed to the widespread and irrational uses
of antibiotics leading to development of
resistance in Staphylococcus aureus.S
imilar findings were seen in a study done by
Aisling et al in an Irish setting who observed
that less than one-third (32.5%) of MRSA urine
samples came from hospital inpatient
sources,implying that MRSA bacteriuria is more
frequently a community-based phenomena.23
The limitations of
the study were that firstly it was laboratory
based study and limited to the cases for which
cultures were requested from the clinic. Secondly,
there was no information on antibiotics
administered prior to sampling for urine culture
or data on subsequent treatment. Thirdly, details
on the method of collection, which has a direct
bearing on urine culture, were not available for
all patients, limiting the analysis of the
pathogens from these samples.
We recommend that a
prospective study on demography of urinary tract
infection is needed to understand the burden of
infection in various age groups and gender along
with the burden of resistant organism with
particular reference to Staphylococcus aureus in
the community.
Acknowledgement
The authors wish to acknowledge staff and
technicians posted in urine bacteriology seat for
their help in preparation of this manuscript.
References
- Flores-Mireles AL, Walker JN, Caparon M,
Hultgren SJ. Urinary tract infections:
epidemiology, mechanisms of infection and
treatment options. Nature Reviews
Microbiology. 2015;13(5):269–284.
- Muthulakshmi M, Gopalkrishnan S. Study on
urinary tract infection among females of
reproductive age group in a rural area of
Kancheepuram district, Tamil Nadu. Int J
Commu Med Public Health 2017;4:3915-3921.
- Kant S, Misra P, Gupta S, Goswami G, Krishnan
K, et al. The Ballabgarh health and demographic
surveillance system (CRHSP-AIMS). Int J
Epidemiol 2018;42: 758-68.
- Medina M, Castillo-Pino E. An introduction to
the epidemiology and burden of urinary tract
infections. Therapeutic Advances in Urology.
2019;11.
- Jhang JF, Kuo HC. Recent advances in recurrent
urinary tract infection from pathogenesis and
biomarkers to prevention. Ci Ji Yi Xue Za
Zhi. 2017;29(3):131-137.
- Kabugo D, Kizito S, Ashok DD et al. Factors
associated with community-acquired urinary tract
infections among adults attending assessment
centre, Mulago Hospital Uganda. African
Health Sciences. 2016;16(4):1131–1142.
- New HC. Urinary tract infections. Am J Med
1996;100 (Suppl.4A): S63-70.
- Jones RN. Impact of changing pathogens and
antimicrobial susceptibility pattern in
treatment of serious infections in hospitalized
patients. Am J Med 1996;100 (Suppl.6A):
S3-12.
- Brosnema DA, Adams JR, Roem CV, Pallares R.
Bacterial pathogens isolated from patients with
blood stream infections. Antimicrobial
agents and Chemotherapy. 1998;42:1762-70.
- Ferry S, Burman LG, Holm SE. Clinical and
bacteriological effects of therapy of urinary
tract infection in primary healthcare: relation
to in vitro sensitivity testing. Scand J
Infect Dis 1988;20:535–44.
- Henry D, Ellison W, Sullivan J, Mansfield DL,
Magner DJ, Dorr MB et al. Treatment of community
acquired acute uncomplicated urinary tract
infection with sparfloxacin versus ofloxacin.
The Sparfloxacin Multi-Center UTI Study Group. Antimicrobial
Agents and Chemotherapy 1998;42:2262–6.
- Najar MS, Saldanha CL, Banday KA. Approach to
urinary tract infections. Indian Journal of
Nephrology. 2009;19(4):129-139
- Manjula NG, Math GC, Patil A, Gaddad SM,
Shivannavar CT. Incidence of Urinary Tract
Infections and Its Aetiological Agents among
Pregnant Women in Karnataka Region. Advances
in Microbiology. 2013;3(6):473-478. doi:
10.4236/aim.2013.36063.
- Stapleton A. Prevention of recurrent
urinary-tract infections in women. Lancet.
1999;353:7–8.
- Jackson SL, Boyko IJ, Scholes D, Abraham L,
Gupta K, Fihn SD. Predictors of urinary tract
infection after menopause. Am J Med. 2004;117:903.
- Mohapatra S, Panigrahy R, Tak V et al.
Prevalence and resistance pattern of
uropathogens from community settings of
different regions: an experience from India.
Access Microbiol. 2022 Feb 9;4(2):000321.
doi: 10.1099/acmi.0.000321.
- Sujatha R, Nawani M. Prevalence of
asymptomatic bacteriuria and it antibacterial
susceptibility pattern. J Clin Diag Res.
2014;8:DCO1-DCO3.
- Christy VR, Athinarayan G, Mariselvam R,
Pallvarnathasamy D, Singh R. Epidemiology of
urinary tract infection in south India. Biomedical
Research and Clinical Practice. 2019;4.10.15761/BRCP.1000190.
- Foxman B. Urinary tract infection syndromes:
occurrence, recurrence, bacteriology, risk
factors, and disease burden. Infect. Dis.
Clin. North Am. 2014;28:1–13.
- Nielubowicz GR, Mobley HL. Host–pathogen
interactions in urinary tract infection.
Nature Rev. Urol. 2010;7:430–441.
- Kline KA, Schwartz DJ, Lewis WG, Hultgren SJ,
Lewis AL. Immune activation and suppression by
group B Streptococcus in a murine model of
urinary tract infection. Infect. Immun. 2011;79:3588–3595.
- Ronald A. The etiology of urinary tract
infection: traditional and emerging pathogens. Am.
J. Med. 2002;113 (Suppl. 1A):14S–19S.
- Looney AT, Redmond EJ, Davey NM, Daly PJ, Troy
C, Carey BF, Cullen IM. Methicillin-resistant
Staphylococcus aureus as a uropathogen in an
Irish setting. Medicine (Baltimore).
2017 Apr;96(14):e4635.
doi:10.1097/MD.0000000000004635.
|



















