Introduction:
Sickle cell hemoglobinopathy is one of the most common monogenic disorders worldwide with an autosomal recessive inheritance (1). Around 300 million sickle cell carriers are present worldwide, with a significant concentration in Africa, the Arab Peninsula, India, the Mediterranean, and the southern United States (2). The sickle trait is prevalent in numerous tribal communities in India, with a prevalence ranging from 1-40 % (3), who generally have a high socioeconomic disadvantage and are medically underserved (4). As per the available literature, many Indian states like Odisha, Andhra Pradesh, Madhya Pradesh, Chhattisgarh have no state-level programs on sickle cell screening and counseling. According to WHO, 70% of deaths due to HbS are preventable by early diagnosis, awareness, and treatment of infections (5). However, the sickle cell trait is rarely associated with symptoms. Hence, counseling and screening programs are most needed to deal with healthy carriers of these traits.
The homozygous form of HbS is associated with very high comorbidities than the heterozygotes (6). This poses enormous stress and financial burden on the families (7). In most developing countries, the healthcare of families having sickle cell hemoglobinopathies is mainly paid through out-of-pocket expenditure (8). Thus, studying the financial impact of sickle cell hemoglobinopathies on households will assist policymakers and health care professionals in developing ways to reduce the burden and identify cost-effective measures in caring for persons with this condition. (9).
Koraput district of Odisha state, where the present study was conducted, is one of the underdeveloped districts and predominantly inhabited by tribal and scheduled caste people, who have a high dominance of socioeconomic disadvantage and are often medically deprived. No studies reported the health care cost of families with sickle cell hemoglobinopathies in this district, and also a very few old studies reported the prevalence of sickle cell carriers in minimal regions. Therefore, the present study aims to determine the prevalence and distribution of sickle cell hemoglobin as well as the economic burden on households in Koraput district of Odisha. Also, the study tries to address the hesitance and stigma towards HbS screening, especially among girls with HbS.
Materials and Methods
A cross-sectional study for sickle cell carrier screening was conducted in Koraput district of Odisha. Initially, a pilot study was carried out in 2 villages, and then the snowball sampling technique was used with the help of villagers, Anganwadi and health workers to locate other villages with a high number of sickle cell carriers. A total of 22 villages, which were remotely situated and where healthcare facilities were not easily accessible, were selected for the present study. Altogether 1092 individuals of either sex, aged above 30 years, were randomly screened from the selected villages (n=22) to assess the prevalence of the sickle cell carrier. After the screening, those found sickling positive, their family members (n=54) were traced and screened to know their HbS status. Blood samples from each individual were collected and tested on the spot to determine the prevalence of sickle cell hemoglobin.
The blood samples were tested by the sodium metabisulphite (Na2S2O5) technique described by Daland and Castle for determining the presence of sickling red cells (10). This method ensures a complete reduction of hemoglobin even in the presence of fetal hemoglobin (11). A 2% Na2S2O5 solution was prepared in sterile distilled water shortly before the tests were started, which were completed in about three hours. One drop of fresh blood was collected on the center of a microscope slide by finger prick with a lancet, and it was immediately mixed with a drop of the metabisulphite solution. The mixture was covered with a coverslip and sealed the edge with nail polish instantly. After incubation for around 30 minutes at room temperature, the slides were studied under a field microscope. The positive cases showed the characteristic sickle shapes of the red cells.
Furthermore, demographic information along with family (n=552) expenditure on healthcare was recorded. Statistical analysis was performed using SPSS (version 22). The study was approved by the Ethical Committee, Department of Anthropology, University of Delhi. During data collection, informed written consent, transcribed in local languages, was obtained from each participant before recruitment.
Results
A total of 1092 individuals were screened for sickle cell trait in Koraput district of Odisha. Of the total samples screened, 508 were males (46.52%) and 584 were females (53.48%). The mean age (SD) of the subjects was 37.5±7.4. 49.5% of the respondents were literate, and the mean educational level was 3.7. Out of 1092 individuals, 103 (9.43%) individuals were found to be sickle cell carriers. Moreover, in the present study, the prevalence of sickle cell trait was found more in females (10.79%) than males (7.87%). Also, the study found a significantly higher prevalence of sickle cell hemoglobins among SCs (9.98%) than STs (3.33%) (Table-1).
Table 1: Prevalence of sickle cell hemoglobin (HbS) in Koraput district of Odisha |
|
No. Screened |
HbS cases |
p-value |
No. |
% |
Total |
1092 |
103 |
9.43 |
Male |
508 |
40 |
7.87 |
0.134 |
Female |
584 |
63 |
10.79 |
Scheduled castes (SC) |
1002 |
100 |
9.98 |
0.05 |
Scheduled tribes (ST) |
90 |
3 |
3.33 |
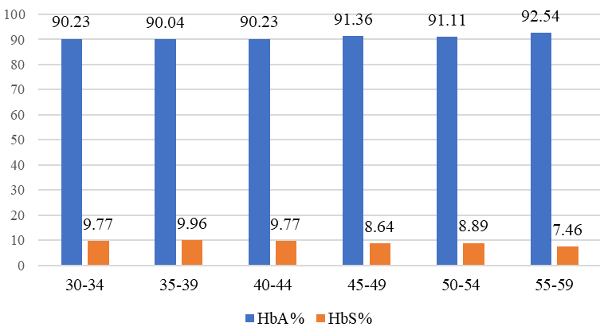 |
Figure 1: Age-wise distribution of HbA and HbS individuals (in %) (p<0.05) |
There found a tendency for the sickling rate to be higher in lower age groups among studied subjects. The highest prevalence (9.96%) was observed among individuals in the 35-39 years age group, whereas the lowest prevalence (7.46%) was in 55-59 years. (Figure-1). 46% of the sickle cell carriers’ parents had a consanguineal marriage.
Table 2 depicts the comparative demographic information of HbA and HbS individuals. A higher number of unmarried and divorced/separated individuals were found among sickle cell carriers (almost 7%). The average annual expenditure on healthcare was found significantly higher in the families having sickle cell hemoglobinopathies, i.e., 3% to almost 65% of the family income. In contrast, the healthcare expenditure was 0.78% to 18.4% in families without sickle cell hemoglobin (p<0.05). The families with only sickle cell carriers had lower spending than in families with sickle cell disease. About 14.3% of the households having HbS had more than 30% healthcare cost, whereas only about 0.6% of the households without HbS had more than 30% healthcare cost (Table 3).
Table 2: Demographic information of HbA and HbS individuals |
Characteristics |
HbA individuals (n=1015) |
HbS individuals (n=131) |
p-value |
Gender |
Male |
478 (47.09%) |
55 (41.98%) |
0.26 |
Female |
537 (52.91%) |
76 (58.02%) |
Age |
Mean age ±SD |
36.42±7.5 |
37.5±7.4 |
|
Community |
SC |
922 (90.80%) |
125 (95.42%) |
0.07 |
ST |
93 (9.16%) |
6 (4.58%) |
Marital status |
Married |
1013 (99.8%) |
124 (94.66%) |
<0.001 |
Single |
2 (0.2) (males) |
8 (6.11%) (5 females & 3 males) |
Divorced/ separated |
0 |
2 (1.53%) |
Education |
Literate |
476 (46.9%) |
60 (45.8%) |
0.81 |
Non-literate |
539 (53.1%) |
71 (54.2%) |
Educational level (mean±SD) |
4.67± 4.32 |
3.7± 3.8 |
|
Family income and healthcare expenditure |
Annual family Income (mean±SD) |
65906 ±13634.11 |
67107±13067.2 |
|
Annual healthcare expenditure (mean±SD) |
2007.18±1164 |
5473.5±1823 |
|
Table 3: Healthcare cost of families with HbA and HbS |
Healthcare expenditure (% of the annual family income) |
No. of households without HbS (n=482) |
No. of households with HbS (n=70) |
p-value |
No. |
% |
No. |
% |
<0.05 |
<10% |
347 |
71.99 |
29 |
41.43 |
10% to <20% |
120 |
24.90 |
20 |
28.57 |
20% to <30% |
12 |
2.49 |
11 |
15.71 |
>30% |
3 |
0.62 |
10 |
14.29 |
Discussion
The objective of screening for sickle cell hemoglobin is to deliver timely care to reduce the morbidity and mortality associated with the disorder. Also, it will help to avoid further inheritance of this disorder to the next generations.
The study found a prevalence of 9.43% of sickle cell traits in Koraput district of Odisha. Similar other studies also reported an almost identical prevalence of sickle cell hemoglobin (12-14). Based on the latest ICMR survey, sickle cell carriers range up to 40% among different groups of India (3). However, no published literature was found that reported the prevalence of sickle cell alleles from the past five decades in the studied area. The prevalence of sickle cell hemoglobinopathy was found to be higher in females than males in the studied population.
The HbS was first reported in a tribal community in southern India (15), leading to the belief that it is limited to tribal communities. Also, several studies stated that sickle cell carriers are more prevalent among the tribal populations (1-35%) (16, 3, 14). However, the present study found the HbS more prevalent in scheduled castes (STs) (9.98%) than in tribal groups (3.33%). This finding corroborates previous studies (12, 17-20).
The present study observed a tendency for the sickling rate to be higher in lower age groups (aged from 59 to 35 years) among studied subjects. The highest prevalence (9.96%) was found among the individuals in the 35-39 years age group, whereas the lowest prevalence (7.46%) was in 55-59 years. This corroborates an earlier study that reported a high prevalence of sickle cell carriers in younger people (21). This difference may be because of the increasing mortality rate with age or the higher number of live births with SCT. The associated comorbidities also rise among sickle cell carriers with aging, which may be the reason behind high mortality among elderly people resulting in a low prevalence of the HbS.
The present study found that about 46% of the sickle cell carriers’ parents had a consanguineal marriage, resulting in the inheritance of the sickle cell trait. An earlier study also described that the prevalence of sickle cell hemoglobinopathies increases in Koraput district because of the consanguineal marriage practice (4). Moreover, a similar study reported a high incidence of sickle cell traits in the Indian subcontinent due to the practice of consanguineal marriage (22). So there exists a lack of awareness and knowledge among them. As the literacy rate among the respondents was relatively lower (46.35%), more awareness requires concerning the inheritance pattern of the sickle cell trait, potential pregnancy issues, HbS screening and counseling. The sickle cell hemoglobin is prevalent in malaria-endemic regions where Plasmodium falciparum was prevalent. Koraput is falls under malaria endemic area (23,24). Therefore, the present study could support the ‘malaria hypothesis’ as the studied area falls under a malaria-endemic zone.
In the studied area, unmarried girls were unwilling to participate in HbS screening. When the reason for this was asked them personally, they described that if they tested positive for the sickle cell hemoglobinopathies, then nobody would marry them. This may be the cause in the present study that more than 6% of the individuals with the HbS were unmarried even after 30 years old, where the mean age at marriage was almost 21 years, similar to the census 2011 (25). Consequently, community-based awareness programs should be initiated to tackle the issues.
The percentage of household income spent as healthcare overheads ranged from 3% to 64.4% in families with sickle cell hemoglobinopathies. In comparison, the expenditure on health in families without sickle cell hemoglobinopathies was 0.78% to 18.4% of their family income. Earlier studies also found a high expenditure rate in families having sickle cell patients (9, 6). However, this proportion affected more in the studied area as they have a comparatively lower family income. The high difference in healthcare costs among families with sickle cell carriers was found because families with only sickle cell carriers had a lower expenditure than families with sickle cell disease. Although recently the government of Odisha has implemented a scheme for providing 500 rupees to each individual with sickle cell disease, it is negligible compared with their healthcare cost. Also, the literacy rate (46.35%) was found to be lower in the studied population as compared to the national (74.04% ) and state (72.9%) level literacy rate (25). It is challenging to manage this disorder and associated issues for people with significantly low literacy and economic status. Therefore, it is high time to implement screening, counseling, and awareness programs in the studied area.
Declarations
Funding:
The authors acknowledge the grants received from the Institution of Eminence, University of Delhi (IoE/FRP/PCMS/2020/27).
Ethics approval: The work was approved by the Ethical Committee, Department of Anthropology, University of Delhi.
Consent to participate: Informed consent was obtained from all patients for being included in the study.
Consent for publication: All authors agreed with the content and that all gave explicit consent to submit.
Conflict of interest:
The authors declare no competing interests.
References
- Serjeant GR, Serjeant BE, editors. Sickle cell disease, 3rd ed. Oxford: Oxford Univ Press; 2001.
- Key NS, Connes P, Derebail VK. Negative health implications of sickle cell trait in high income countries: from the football field to the laboratory. British Journal of Haematology. 2015; 170(1): 5-14.
- Colah RB, Mukherjee MB, Martin S, Ghosh K. Sickle cell disease in tribal populations in India. The Indian Journal of Medical Research. 2015; 141(5): 509.
- Bindhani BK, Devi NK, Nayak JK. Knowledge, awareness, and attitude of premarital screening with special focus on sickle cell disease: a study from Odisha. Journal of Community Genetics. 2020; 11(4): 445-449.
- Williams TN, Obaro SK. Sickle cell disease and malaria morbidity: a tale with two tails. Trends in Parasitology. 2011; 27(7): 315-320.
- Ngolet LO, Engoba MM, Kocko I, Dokekias AE, Mombouli JV, Moyen GM. Sickle-cell disease healthcare cost in Africa: Experience of the Congo. Anemia. 2016.
- Brown BJ, Okereke JO, Lagunju IA, Orimadegun AE, Ohaeri JU, Akinyinka. Burden of healthcare of carers of children with sickle cell disease in Nigeria. Health & Social Care in the Community 2010; 18(3): 289-295.
- Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJ. Household catastrophic health expenditure: a multicountry analysis. The Lancet. 2003; 362(9378): 111-117.
- Olatunya OS, Ogundare EO, Fadare JO, Oluwayemi IO, Agaja OT, Adeyefa BS, Aderiye O. The financial burden of sickle cell disease on households in Ekiti, Southwest Nigeria. Clinico Economics and Outcomes Research: CEOR. 2015; 7:545–553.
- Daland GA, Castle WB. A simple and rapid method for demonstrating sickling of the red blood cells: the use of reducing agents. The Journal of Laboratory and Clinical Medicine. 1948; 33(9): 1082-1088.
- Allison AC. Notes on sickle-cell polymokphism: with statistical appendix, by Sheila Maynard Smith. Annals of Human Genetics. 1954; 19(1): 39-51.
- Colah R, Mukherjee M, Ghosh K. Sickle cell disease in India. Current Opinion in Hematology. 2014; 21(3): 215-223.
- Patel J, Patel B, Gamit N, Serjeant GR. Screening for the sickle cell gene in Gujarat, India: a village-based model. Journal of Community Genetics. 2013; 4(1): 43-47.
- Patra PK, Chauhan VS, Khodiar PK, Dalla AR, Serjeant GR. Screening for the sickle cell gene in Chhattisgarh state, India: an approach to a major public health problem. Journal of Community Genetics. 2011; 2(3): 147-151.
- Lehmann H, Cutbush M. Sickle-cell trait in southern India. British Medical Journal. 1952; 1(4755): 404.
- Bindhani BK, Nayak JK. Quality of life among individuals with sickle cell disease: A study from Koraput district, Odisha. International Journal of Academic Research and Development. 2018; 3(2): 726-730.
- Charuhas AV, Neelam SD, Sanjay KS, Sanjay AB, Mohan KB, Sanjeev CM, Manjusha DA. Do gender differences influence the prevalence of sickle cell disorder and related morbidities among school children in rural central India?. International Journal of Collaborative Research on Internal Medicine & Public Health. 2013; 5(5): 348.
- Mohanty D, Das K. Genetic counselling in tribals in India. The Indian Journal of Medical Research. 2011; 134(4): 561.
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, Weatherall DJ, Hay SI. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nature Communications. 2010; 1(1): 1-7.
- Saha N, Banerjee B. Incidence of abnormal hemoglobins in different ethnic groups of India. Hum Genetik. 1971; 11(4):300-3.
- Kar BC, Devi S, Dash KC, Das M. The sickle cell gene is widespread in India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987; 81(2): 273-275.
- Mukherjee BN, Das MK. Spatial distribution of two predominant abnormal haemoglobins – HbE and HbS in Indian subcontinent. J. Indian Anthrop. Soc. 1990; 25: 39-59.
- Rajagopalan PK, Pani SP, Das PK, Jambulingam P. Malaria in Koraput district of Orissa. The Indian Journal of Pediatrics. 1989; 56(3): 355-364.
- Das LK, Padhi B, Sahu SS. Prediction of outcome of severe falciparum malaria in Koraput, Odisha, India: A hospital-based study. Tropical Parasitology. 2014; 4(2): 105–110.
- District Census Handbook, Koraput. Census of India-2011. https://censusindia.gov. in/2011census/dchb/2129_PART_B_DCHB_KORAPUT.pdf. Accessed September 11th, 2021.
|

