|
Introduction
Filariasis
are tissue helminthiases caused by white filiform
worms or nematodes transmitted by arthropods
(diptera: mosquitoes, flies and midges) (1). Nine
filarial species have been described in humans.
Depending on their impact, they are divided into
pathogenic filariases (onchocerciasis, loasis,
brugiosis, wucheriosis or brancoftosis) and low-
or non-pathogenic filarioses (M. perstans, M.
rodhaini, M. ozzardi and M.
streptocerca mansonellosis) (2). In Gabon,
5 species are encountered: L. loa, M.
perstans, O. volvulus, M. streptocerca and M.
rodhaini (3). Because of their chronicity,
these filariases can become systemic diseases,
affecting organs such as the heart, lungs, kidneys
and liver, and leading to a dysregulation of the
host's immune response (4). With almost a billion
people exposed to these parasites and over 200
million affected, they are widespread throughout
the world. Because of the disability they cause
among African workers, the people living in
high-risk areas have been estimated at nearly 14.4
million cfa francs (5). Loa loa
filariasis is a parasitosis endemic to the
tropical forests of Central and West Africa. It is
transmitted by a hematophagous female fly of the
genus Chrysops (6). More than 15-20
million people are infected with L. loa (7). In
endemic countries such as Cameroon, Central
African Republic, Congo, Democratic Republic of
Congo, Gabon and Equatorial Guinea, Loa loa
filariasis is a real public health problem. After
malaria, it is the second most common cause of
parasitological consultations. It is also found in
endemic areas in southern Nigeria, Chad, Sudan and
on its border with Kenya, as well as in Angola
(8).
Loa loa
filariasis has received little attention and is
not included in the list of neglected tropical
diseases such as lymphatic filariasis and
onchocerciasis (6). It attracts attention mainly
because of the embarrassing symptoms it manifests,
such as pruritus, migration of adult worms into
the eye and so-called Calabar edema (9). It
contraindicates the treatment of "river
blindness", also known as onchocerciasis, in cases
of coinfection due to severe, even fatal,
neurological reactions (10). However, in 2016, it
was shown that this pathology is more dangerous
than it appears. In fact, in infected people over
the age of 24 with a parasitaemia greater than or
equal to 30,000 mf/ml of blood, it would increase
the risk of mortality (7). Also, in cases of
hyperparasitemia of Loa loa filariasis,
serious adverse effects can occur during treatment
with diethylcarbamazine (DEC) and ivermectin,
requiring close monitoring of treatment that
prevents the mass administration of antifilarial
drugs, aimed at controlling other filaria in areas
where Loa loa is coendemic (11).
Numerous epidemiological studies on Loa
loa filariasis, have been conducted
throughout the West and Central African forest
block. They have indicated Cameroon as the most
important focus of the disease, with a prevalence
of over 50% in four localities (12). In Gabon, a
country covered by an immense tropical forest,
despite the existence of a study that revealed a
prevalence of Loa loa filariasis of
22.4% in 4392 individuals aged at least 15
throughout the country (3) and a more recent one,
which reported a prevalence of 9.27% (95% CI:
[0.05. 0.15]) among pregnant women at the
Sino-Gabonese Friendship Hospital in Franceville
(13), the benign nature attributed to Loa loa
filariasis contributes to making this disease a
public health problem in the country. It is in
this context that this study was undertaken, with
the overall aim of determining the prevalence and
risk factors associated with Loa loa
filariasis in South-eastern Gabon.
Materials and Methods
Presentation of the study and sampling
site:
Sampling for the
present study was carried out at the Paul MOUKAMBI
Regional Hospital in Koula-Moutou (PMRHKM) in the
Mikoumou district of Koula-Moutou (Central-eastern
Gabon). This healthcare institution is located in
the MIKOUMOU district, at a latitude of .1.12724°
or 1°7'38'' South and a longitude of 12.47764° or
12°28'40'' in the North-east of the town of
Koula-Moutou, capital of the Ogooué-Lolo province.
It is located at the entrance to the town from
Lastourville, 800 m from the crossroads on the
paved road. Inaugurated in 2002 by President OMAR
BONGO, the PMRHKM comprises 15 buildings housing
several departments, including the laboratory.
Koula-Moutou is a semi-urban town characterized by
alternating dwellings and equatorial vegetation.
Human activities are based on administrative work,
hunting, fishing, agriculture, trade and so on.
The Koula-Moutou-Lastourville and
Koula-Moutou-Pana axes are characterized by the
presence of large expanses of equatorial
vegetation all along the road, and human
activities are based on forestry work, hunting,
fishing, agriculture and so on.
|
Fig
1: Study Site Map
|
Type, period
and population of the study
Conducted from April
03 to July 31, 2023, this prospective,
cross-sectional, analytical study involved
randomly selected men and women aged at least 15
years, who had come during the study period to be
diagnosed for Loa loa filariasis at the
biomedical analysis laboratory of the PMRH in
Koula-Moutou, and who met the inclusion criteria.
Inclusion
and exclusion criteria for study participants:
Only people who
- Were at least 15 years old
- Had consented to participate in the study
- Had agreed to complete the survey
questionnaire
were included.
People who did not
wish to take part in the study, or whose
examination results were unusable, were excluded.
Determining
sample size
To estimate the
prevalence and risk factors associated with Loa
loa filariasis in the town of Koula-Moutou
and surrounding departments, the study sample size
was determined using the single-proportion
population formula as used elsewhere, positing the
formula:
n = (Zα / 2)2
X (P, (1 - P)2 / (d)2) (14)
In this, n
represents the number of the sample size, Z α /2
is the standard normal deviation (1.96)
corresponding to a 95% confidence interval (CI), P
is the prevalence of Loa loa filariasis.
In the absence of P values obtained elsewhere, or
in previous studies in the town of Koula-Moutou
and surrounding departments, this P value was
taken to be 50%. d is taken to be the
precision/marginal error (d = 0.05) or 5%.
Initially, the sample size determined for the
study was 273. As it has been applied in two
studies elsewhere, errors resulting from the
probability of non-compliance or abandonment were
minimized, the sample size was increased by 10%
(15,16). Finally, the sample size used in the
present study was 300 participants.
Sample
collection
After signing the
informed consent, venous blood samples (at the
elbow crease) were taken in EDTA tubes between 11
am and 3 pm (due to daytime activity) at the
laboratories of the PMRH in Koula-Moutou.
EDTA
tube:
This is a
purple-capped tube containing an anticoagulant:
Ethylene Diamine Tetra Acetic (EDTA) and a calcium
chelator (which lowers calcium). Plasma is
obtained from the EDTA tube. This is the tube par
excellence for CBC, CV, GE, RMF and PCR.
Parasitological
diagnosis of Loa loa filariasis
Loa loa
filariasis is diagnosed using two techniques:
fresh blood and leucoconcentration. Detection of
the parasite as an adult or in the microfilarial
stage provides a definitive confirmation of
infection. Adult filariae are rarely found; they
can be found and extracted as they pass under the
conjunctiva or skin. A blood sample is used to
test for microfilariae.
Direct
examination of fresh blood: 10 µL
of whole blood is placed between a slide and a
coverslip and observed with a light microscope at
x10 and x40. Microscopic observation of fresh
blood enables the presence of microfilariae to be
detected thanks to their rapid movements. Indeed,
Loa loa is a long worm with low mobility
(diurnal) at the PMRH laboratories in
Koula-Moutou.
Concentration
techniques: Also known as the
leucoconcentration technique, this is performed if
the direct examination is negative and is carried
out as follows:
Decantation
Whole blood (EDTA)
is centrifuged at 2000 rpm for 5 minutes, then the
supernatant (plasma) is removed.
Hemolysis
1
Add 2 mL saline (9%
NaCl) and 1 mL saponin (2%), then invert the tube
successively and leave for 10 minutes. Then
centrifuge at 2000 rpm for 10 minutes and discard
the supernatant.
Hemolysis
2
Add 2 mL saline (9%
NaCl) and 1 mL saponin (2%), then successively
invert the tube and centrifuge at 2000 rpm for 5
minutes, discarding the supernatant.
Washing
Add 3mL of saline
(9% NaCl) to the pellet, successively invert the
tube and centrifuge at 2000 rpm for 10 minutes,
then discard the supernatant and drain.
Reading
Place the pellet
between slide and coverslip and read under the
microscope at x10 and x40.
Other
parasitological diagnostics are also performed,
such as:
- Thin smear and thick drop tests: These are
used to identify microfilariae on the basis of
characteristics specific to each species (size,
coloring or absence of the sheath, appearance
and size of nuclei, etc.).
- Blood microfilaria count: useful before
starting treatment with a microfilaricide.
Survey
questionnaire
Patients were
interviewed to collect data on potential risk
factors for Loa loa filariasis using a
structured questionnaire, covering
socio-demographic information, such as age,
marital status, instruction level, professional
status, residence (Urban or rural)), residence
duration, and housing conditions. In addition,
behavioral risk factors and medical history of
study participants, such as tattooing, blood
transfusion, surgery, clinical signs, were
addressed
Quality
assurance
Using standardized
data collection tools, data quality was ensured by
pre-testing questionnaires on 5% of participants,
after appropriate training of staff in data
collection and management of an integrated quality
control system at the Paul MOUKAMBI Regional
Hospital in Koula-Moutou (PMRHKM). All laboratory
procedures were carried out in accordance with
standard operating procedures.
Ethical
considerations:
Ethical
authorization was obtained from the Director
General of the Centre Hospitalier Régional Paul
MOUKAMBI de Koula-Moutou, which maintains
partnerships with the USTM Faculty of Sciences. An
internship agreement was obtained from the Dean of
the Faculty of Science to conduct the study in the
said structure. Written informed consent was
obtained from each participant, who was informed
in advance of the right to terminate participation
in the present study at any time. Confidentiality
of information was maintained by coding and
storage in a lockable cabinet. Clinicians were
informed of the results for patient management.
Statistical analysis:
Data collected were
entered into a Microsoft Excel 2013 spreadsheet,
cleaned and then analyzed with R software version
3.6.1. Pearson's chi-square, Odds ratios, and 95%
confidence intervals were used to find potential
associations between risk factors and Loa loa
filariasis. P-values were determined and
considered significant when less than or equal to
0.05.
Results
Prevalence
of Loa loa filariasis among study
participants (N =300).
A total of 300
participants were registered for this study. With
a sex ratio (M/F) of 0.60, women were in the
majority. With a mean age of 26.7 ± 17.45 years,
the majority of study participants, 60 (20%), were
aged between 31 and 40 years, and 188 (62.67%)
were women. Diagnosis for Loa loa
filariasis indicated that 42 participants suffered
from this disease, a prevalence of 14% (95% CI:
[0.10. 0.18]), compared with 86%, or 258 negative
results [Figure 2].
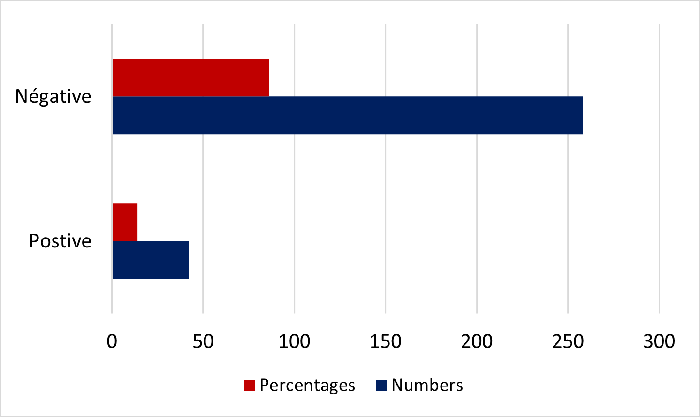
|
| Figure
1 : Prevalence of Loa loa
filariasis among study participants (N
=300). |
Prevalence
of Loa loa filariasis according to
socio-demographic characteristics of the study
population, (N=300).
Statistically
significant associations between dependent and
independent variables were found in univariate
analyses of the prevalence of Loa loa
filariasis according to sociodemographic
characteristics. Among Loa loa
filariasis-positive patients in the study, those
aged between 61 and 70 years (OR = 2.66, 95% CI :
[1.09 ; 6.48] p =0.003*) , female (OR = 2.58, 95%
CI : [1.33 ; 5.01] p =0.042* ), retired (e) (OR =
3.35, 95% CI : [1.08; 10.35] p =0.03*), living in
rural areas (OR = 6.39, 95% CI: [3.06; 13.35] p
<0.0001), living in average conditions (OR =
5.15, 95% CI: [2.54; 10.43] p <0.001), were
statistically associated with Loa loa
filariasis Further study using multivariate
logistic regression of the variables indicated
that women (adjusted OR= 56.9, 95% CI: [1.71;
1893.6] p =0.024*) were fifty-seven times more
likely to suffer from Loa loa filariasis
(Table 1).
|
Table 1: Univariate and
multivariate analyses of the prevalence
of Loa loa filariasis,
according to socio-demographic
characteristics of study participants
(N=300).
|
|
Variables
|
Total number of people diagnosed
N (%)
|
Prevalence of Loa loa
filariasis
|
Univariate analysis
|
Multivariate analysis
|
|
Positive N (%)
|
Negative N (%)
|
Crude OR CI 95%
|
p
|
Adjusted OR CI 95%
|
p
|
|
Age groups (years)
|
|
≤ 20
|
44 (14.67)
|
6 (13.63)
|
38 (86.37)
|
0.96
[0.38 ; 2.43]
|
0.94
|
-
|
-
|
|
21 - 30
|
56 (18.67)
|
4 (7.14)
|
52(92.86)
|
Reference
|
-
|
-
|
-
|
|
31 - 40
|
60 (20)
|
5 (8.33)
|
55 (91.67)
|
0.5
[0.19 ; 1.33]
|
0.16
|
-
|
-
|
|
41 - 50
|
47 (15.67)
|
4 (8.51)
|
43 (91.49)
|
0,53
[0.18 ; 1.56]
|
0.24
|
-
|
-
|
|
51 - 60
|
29 (9.67)
|
6 (20.69)
|
23 (79.31)
|
1.17 [0.65 ; 4.46]
|
0.28
|
-
|
-
|
|
61 - 70
|
29 (9.67)
|
8 (27.59)
|
21 (72.41)
|
2.66 [1.09 ; 6.48]
|
0.027*
|
-
|
-
|
|
71 - 80
|
20 (6.67)
|
5 (25)
|
15 (75)
|
2.19 [0.75 ; 6.38]
|
0.14
|
-
|
-
|
|
≥ 81
|
15 (4.98)
|
4 (26.67)
|
11 (73.33)
|
2.64 [0.8 ; 8.76]
|
0.15
|
-
|
-
|
|
Gender
|
|
Male
|
112 (37.33)
|
24 (21.42)
|
88 (78.58)
|
Reference
|
|
1
|
|
|
Female
|
188 (62.67)
|
18(9.57)
|
170 (90.43)
|
2.58 [1.33; 5.01]
|
0.042*
|
56.9 [1.71; 1893.6]
|
0.024*
|
|
Marital status
|
|
Married
|
43 (14.33)
|
9 (20.93)
|
34 (79.07)
|
Reference
|
-
|
-
|
-
|
|
Single
|
117 (29)
|
12 (10.26)
|
105 (89.74)
|
0.58 [0.28; 1.18]
|
0.14
|
0.64 [0,02; 239.9]
|
0.89
|
|
Cohabiting
|
132 (44)
|
20 (15.15)
|
112 (84.85)
|
2 [1.05; 3.81]
|
0.61
|
8,8 [0,12; 128.3]
|
0.51
|
|
Widowed
|
8 (2.67)
|
1 (12.5)
|
7 (87.5)
|
0.87 [0.1; 7.26]
|
0.90
|
-
|
-
|
|
Professional status
|
|
Civil servant
|
41 (13.67)
|
6 (14.63)
|
35 (85.37)
|
Reference
|
|
1
|
-
|
|
Pupil/Student
|
67 (22.33)
|
14 (20.9)
|
53 (79.1)
|
1,93 [0.95; 3.92]
|
0.065
|
-
|
-
|
|
Unemployed
|
86 (28.67)
|
8(9.30)
|
78 (90.7)
|
0.56 [0.25; 1.27]
|
0.16
|
-
|
-
|
|
Farmer
|
91 (30.33)
|
9 (9.89)
|
82 (90.11)
|
0,59 [0.27; 1.29]
|
0.18
|
4.46 [0.075; 259.7]
|
0.471
|
|
Retired
|
15 (5)
|
5 (33.33)
|
10 (66.67)
|
3.35 [1.08; 10.35]
|
0.03*
|
-
|
-
|
|
Instruction level
|
|
Illiterate
|
41 (13.67)
|
2 (4.88)
|
39 (95.12)
|
Reference
|
|
1
|
|
|
Primary
|
70 (23,33)
|
19 (27.14)
|
51 (72.86)
|
3.35 [1.7; 6.62]
|
0.000*
|
0.722 [0.001; 654.5]
|
0.925
|
|
Secondary
|
168 (56)
|
20 (11.90)
|
148 (88.1)
|
0.45 [0.23; 0.87]
|
0.36
|
-
|
-
|
|
University
|
21 (7)
|
1(4.76)
|
20 (95.24)
|
0.56 [0.13; 2.48]
|
0.21
|
-
|
-
|
|
Residence
|
|
Koula-Moutou (semi-rural)
|
190 (63.33)
|
11 (5.79)
|
179 (94.21)
|
Reference
|
‘
|
1
|
-
|
|
Other (rural)
|
110 (36.67)
|
31(28.18)
|
79 (71.82)
|
6.39 [3.06; 13.35]
|
<0.0001
|
-
|
-
|
|
Resident for (years)
|
|
≤ 10
|
82
(27.33)
|
6 (7.32)
|
76 (92.68)
|
2.51 [1.02 ; 6.2]
|
0.63
|
]
|
-
|
|
≥ 10
|
218
(72.67)
|
36
(16.51)
|
40
(83.49)
|
Reference
|
|
1
|
|
|
Housing conditions
|
|
Average
|
117 (39)
|
39 (33.33)
|
78 (66.67)
|
5.15 [2.54; 10.43]
|
<0.001
|
-
|
-
|
|
Good
|
183 (61)
|
3 (35.57)
|
180 (64.43)
|
Reference
|
-
|
1
|
-
|
|
OR = odds ratio; CI= confidence interval;
* = significant test
|
History and
clinical and paraclinical aspects associated
with the prevalence of Loa loa
filariasis in study participants. (N = 300)
To test the
association between Loa loa filariasis
exposure and the medical history and clinical
signs of the study participants, crude and
multivariate logistic regression analyses of the
variables were carried out. It was found that only
study participants with clinical signs such as
pruritus, dermatitis or Calabar edema (OR = 0.16,
95% CI: [0.08; 0.32] p <0.0001) were at greater
risk of developing Loa loa filariasis
than other participants. Multivariate logistic
regression, on the other hand, indicated no
statistically significant association between
participants' clinical signs or medical history
and Loa loa filariasis Table 2.
|
Table 2: Univariate and
multivariate logistic regression
analysis of the prevalence of Loa
loa filariasis, according to the
medical history and clinical signs of
study participants (N = 300).
|
|
Variables
|
Total number of people diagnosed
N (%)
|
Prevalence of Loa loa
filariasis
|
Univariate analysis
|
Multivariate analysis
|
|
Positive N (%)
|
Negative N (%)
|
Crude OR CI
95%
|
p
|
Adjusted OR CI
95%
|
p
|
|
Tattoo
|
|
Yes
|
56 (22.95)
|
7 (12.5)
|
49 (87.5)
|
Reference
|
|
1
|
|
|
No
|
244 (77.95)
|
35 (14.34)
|
209 (85.66)
|
0.85 [0.36; 2.03]
|
0.89
|
-]
|
-
|
|
History of surgery
|
|
Yes
|
44 (14.67)
|
11 (25)
|
33 (75)
|
1.97 [0.92; 4.23]
|
0.122
|
-
|
-
|
|
No
|
256 (8.33)
|
31 (12.11)
|
225 (87.89)
|
Reference
|
-
|
1
|
-
|
|
History of blood transfusion
|
|
Yes
|
11 (3.67)
|
4 (36.36)
|
7 (63.64)
|
3.77 [1.05; 13.49]
|
0.08
|
-
|
-
|
|
No
|
289 (96.33)
|
38 (13.15)
|
251 (86.85)
|
Reference
|
-
|
1
|
-
|
|
|
|
Clinical signs (pruritus,
dermatitis, Calabar edema)
|
|
Yes
|
117 (39)
|
25 (21.37)
|
92 (78.63)
|
0.16 [0.08; 0.32]
|
<0.001
|
1.9 [0.13; 26.32]
|
0.65
|
|
No
|
183 (61)
|
17 (9.29)
|
166 (90.71)
|
Reference
|
-
|
1
|
-
|
|
OR = odds ratio; CI= confidence interval;
* = significant test
|
Discussion
Highly endemic in
Central African countries such as Gabon, and the
cause of a high disease burden in these countries,
Loa loa filariasis is a serious human
parasitosis. In order to design, plan and evaluate
appropriate intervention strategies against this
disease, knowledge of its epidemiology,
transmission, distribution and extent, as well as
the associated risk factors, is required (17).
With the main objective of determining the
prevalence and risk factors associated with Loa
loa filariasis: case of Koula-Moutou and
surrounding departments, South-central Gabon, The
present study reported a prevalence of Loa
loa filariasis of 14% (95% CI: [0.10-
0.18]), This result is similar to that found in a
previous study (18). However, it is higher than
that obtained by a study elsewhere, which found an
average prevalence of L. loa in the
village of 6.3% (19), and lower than that of
another study, conducted in Cameroon, which found
an overall prevalence of loasis of 27.3% (20) and
that of another study conducted in another region
of Gabon, a prevalence of Loa loa
microfilaremia of 22.4% was reported in one study
(3). The variability of these results could be
explained, on the one hand, by the number of
participants recorded in each study and the
diagnostic methods used. On the other hand,
knowing that there is a periodicity of Loa
loa microfilaremia (21), the difference in
periods, regions and years of the studies may
influence these results.
Contrary to studies
that reported that the 18-28 age group was
statistically more associated with filariasis than
other age groups (22), or that the maximum load of
Loa loa microfilariae, was found in
individuals aged 35-49 years (20), univariate
analyses of the prevalence of Loa loa
filariasis according to sociodemographic
characteristics, history, medical, and clinical
signs of study participants, indicated that being
aged between 61 and 70 years, was significantly
associated with Loa loa filariasis. This
finding is in line with a study that indicated
that the prevalence of Loa loa
microfilaremia was highest (14.3%) in people aged
between 65 and 84 years (18). This may reflect
this age group's greater exposure to chrysop
bites than younger groups. Most elderly people are
also exposed to a number of factors which may
contribute to their predisposition to infections,
such as impaired immune function (23).
Contrary to the
results obtained in previous studies, which
reported that men were likely to have a higher
prevalence of Loa loa filiarisis than
women (24), a statement also supported by
epidemiological data from central Cameroon, where
the prevalence of Loa loa filariasis was
significantly higher in men than in women (25), in
the present study, he reported that factors such
as female gender, widow, retired, living in rural
areas, housed in average conditions, were
significantly associated with Loa loa
filariasis. This result, confirmed by multivariate
logistic regression analysis, is in line with a
study carried out elsewhere, in which a female was
more likely to report a history of eyeworm than a
male subject (26). This can be justified by the
fact that, in the African context, even a widow,
the woman is the pillar of the family, and the
domestic chores performed by the latter constitute
so many challenges to their health that weigh on
their perception of it. They may also deteriorate
their functional health, due to the physical
efforts required (27). The women in the present
study, residing in rural areas and housed in
average conditions, were significantly associated
with Loa loa filariasis. Now retired,
their main activities were farming and fishing,
which are carried out in forests. This result,
although contrary to that of a study on the
subconjunctival and intraocular presence of adult
Loa loa in populations living in urban
centers (28), is however, in line with a study
that indicated that Loa loa and other
filariases are established diseases observed in
villages and rural communities in endemic areas
(12). In addition, most homes are surrounded by
vegetation or forest, potential reserve for Chrysops,
the loase vector (22). In the present study, it
was reported that only study participants with
clinical signs such as pruritus, dermatitis or
Calabar edema were more likely to develop Loa
loa filariasis than other participants.
This result is in line with that of a study
conducted in Burkina Faso, in which transient
angioedema (Calabar edema) and pruritus were
observed in a patient diagnosed with Loa loa
filariasis (29). Multivariate logistic regression
showed no statistically significant association
between clinical signs or medical history of
participants and Loa loa filariasis.
Study limitations
Despite the contributions made, the present study
has certain limitations that should be highlighted
for future studies. Within the conceptual
framework of this study, a number of factors were
identified upstream of the living context of the
Koula-Moutou populations and the socio-demographic
characteristics likely to influence Loa loa
filariasis. Firstly, clinical signs based on a
questionnaire are highly subjective. Blood smear
staining and concentration techniques used in this
study are limited. Detection of Loa loa
filariasis in peripheral blood is insensitive, as
only 30% of individuals are microfilaremic, while
70% are amicrofilaremic with a variety of clinical
signs (30). In the presence of an apparently
healthy individual with occult infections, the
negative serological screening test could be
biased. The application of nucleic acid-based
detection techniques, such as polymerase chain
reaction (PCR), reverse transcriptase PCR
(RT-PCR), loop-mediated isothermal amplification
and lateral flow assays (LFA), or the detection of
biomarkers, such as immunoglobulin 4 (IgG4),
antibodies directed against Loa loa
antigens, would have been appropriate to prevent
infection in the participants of the present
study.
Conclusion and Outlook
By providing key
information, the results of the present study show
very active transmission of Loa loa
filariasis in Koula-Moutou and surrounding
departments, as suggested by the prevalence rate.
The latter was clearly associated with
socio-demographic variables of the study
participants, such as being aged between 61 and
70, female, retired, living in rural areas, living
in average conditions and presenting clinical
signs such as pruritus, dermatitis or Calabar
swelling. These results could guide Gabonese
health authorities in the control and prevention
of Loa loa filariasis. To implement
appropriate intervention strategies against Loa
loa filariasis, Gabonese health authorities
should introduce molecular (qPCR) and
immunological diagnostic methods, such as the use
of biomarkers, which could help identify the true
profile of Loa loa infection, beyond
pathognomonic signs such as the presence of
microfilariae or attested ocular passage, specific
but not sensitive enough to detect all clinical
cases; in this case, biomarkers may be useful in
areas where this condition is endemic.
Acknowledgments
We would like to
thank all the participants of this study, the
Regional Health Department of the Centre-East, in
Koula-Moutou. We also thank, the management of the
Paul Moukambi regional hospital in Koula- Moutou,
and the staff of the medical analysis laboratory,
for their availability.
References
- Sarwar M. Typical flies: Natural history,
lifestyle and diversity of Diptera. In Life
Cycle and Development of Diptera.
IntechOpen. 2020
- Jones-Sheets MA, Chen M, Cruz JC. Cutaneous
Filariasis in an American Traveler. Cutis,
2020;106(4): E12-E16.
- Akue JP, Nkoghe D, Padilla C et al.
Epidemiology of Concomitant Infection Due to Loa
loa and Mansonella perstans in
Gabon. PLoS. 2011;5(10):e1329
- Wanji S, Ndongmo C, Fombad WP et al. Impact of
repeated annual community treatment with
ivermectin on parasitological indicators of
loasis in Cameroon: implications for the
elimination of onchocerciasis and lymphatic
filariasis in Loa loa co-endemic areas
in Africa.. PLoS Neglected Tropical
Diseases. 2018;12(9):e0006750.
- Zoure HGM, Wanji S, Noma M et al. The
geographic distribution of Loa loa in
Africa: results of large-scale implementation of
the Rapid Assessment Procedure for Loiasis
(RAPLOA). PLoS Neglected Tropical Diseases.
2011;5(6):e1210.
- Pallara E, Cotugno S, Guido G et al. Loa
loa in the Vitreous Cavity of the Eye: A
Case Report and State of Art. Am J Trop Med
Hyg. 2022;107(3):504–16. Available at:
https://doi.org/doi: 10.4269/ajtmh.22-0274.
- Chesnais CB, Takougang I, Paguele M, Pion SD,
Et Boussinesq M. Excess mortality associated
with loiasis: a retrospective population-based
cohort study. The Lancet Infectious
Diseases. 2016;17(1):108-116.
- Kobayashi T, Hayakawa K, Mawatari M et al.
Loiasis in a Japanese Traveler Returning from
Central Africa. Tropical Medicine and
Health. 2015;43(2):149–53. Available at:
https://doi.org/doi: 10.2149/tmh.2015-05
- Barrett MP, Giordani F. Inside Doctor
Livingstone: a Scottish icon meets a tropical
disease. Parasitology.
2017;144(12):1652–1662. Available at:
https://doi.org/doi : 10.1017/S003118201600202X
- Vinkeles Melchers NVS, Coffeng LE, Boussinesq
M et al. Projected Number of People with
Onchocerciasis-Loiasis Coinfection in Africa,
1995 to 2025. Clin Infect Dis.
2020;23;70(11):2281–2289. Available at:
https://doi.org/doi: 10.1093/cid/ciz647.
- Herrick JA, Legrand F, Gounoue R et al.
Post-treatment Reactions after Single-Dose
Diethylcarbamazine or Ivermectin in Subjects
with Loa loa Infection. Clin Infect
Dis. 2017;15;64(8):1017–1025. Available
at: https://doi.org/doi: 10.1093/cid/cix016.
- Dieki R, Nsi-Emvo E, Akue JP. The Human Filai
Loa loa: Update on Diagnostics and
Immune Response. Res Rep Trop Med. 2022
Aug 1;13:41-54. doi: 10.2147/RRTM.S355104. PMID:
35936385; PMCID: PMC9355020
- Adelaïde N, Sima CO, Mba TN et al.
Prevalence of Loa loa filariasis among
pregnant women seen at the Sino-Gabonese
Friendship Hospital in Franceville in 2022. International
Journal of Current Science (IJCSPUB).
2023;13(2).
- Daniel W. W. Biostatistics a Foundation for
Analysis in the Health Science (9th ed.) New
York: John Willey and Sons Inc, USA; 2009
- Tadesse G. The prevalence of intestinal
helminthic infections and associated risk
factors among schoolchildren in the town of
Babile, eastern Ethiopia. The Ethiopian
Journal of Health Development. 2005;19
(2):140–147. doi: 10.4314/ejhd.v19i2.9983
- Sitotaw B, Mekuriaw H, Damtie D. Prevalence of
intestinal parasitic infections and associated
risk factors in children at Jawi Primary School,
Jawi City, Northwest Ethiopia. BMC
Infectious Diseases. 2019;19(1):341.
doi : 10.1186/s12879-019-3971-x.
- Kelly-Hope L, Paulo R, Thomas B, Brito M,
Unnasch TR, Molyneux D. Loa loa vectors Chrysops
spp.: perspectives on research, distribution,
bionomics, and implications for elimination of
lymphatic filariasis and onchocerciasis. Parasit
Vectors. 2017 Apr 5;10(1):172. doi:
10.1186/s13071-017-2103-y. PMID: 28381279;
PMCID: PMC5382514
- Ojurongbe O, Akindele Aa, Adeleke Ma, Oyedeji
Mo, Adedokun Sa, Ojo JF. Co-endemicity of
loiasis and onchocerciasis in rain forest
communities in South western Nigeria. PLoS
Negl Trop Dis. 2015 Mar 26;9(3):e0003633.
doi: 10.1371/journal.pntd.0003633. PMID:
25812086; PMCID: PMC4374772.
- Emukah E, Rakers LJ, Kahansim B et al. In
Southern Nigeria Loa loa Blood
Microfilaria Density is Very Low Even in Areas
with High Prevalence of Loiasis: Results of a
Survey Using the New Loa Scope Technology. Am
J Trop Med Hyg. 2018 Jul;99(1):116-123.
doi: 10.4269/ajtmh.18-0163. Epub 2018 May 10.
PMID: 29761763; PMCID: PMC6085777.
- Mogoung-Wafo AE, Nana-Djeunga HC, Domche A,
Fossuo-Thotchum F, Bopda J, Mbickmen-Tchana S.
Prevalence and intensity of Loa loa
infection over twenty-three years in three
communities of the Mbalmayo health district
(Central Cameroon). BMC Infect Dis. 2019
Feb 13;19(1):146. doi:
10.1186/s12879-019-3776-y. PMID: 30760228;
PMCID: PMC6373160.
- Campillo JT, Louya F, Bikita P, Missamou F,
Pion SDS, Boussinesq M, Chesnais CB. Factors
associated with the periodicity of Loa loa
microfilaremia in the Republic of the Congo. Parasit
Vectors. 2022 Nov 9;15(1):417. doi:
10.1186/s13071-022-05541-y. PMID: 36352480;
PMCID: PMC9647901.
- Kenguele M.H, Meye B, Ndong M T, Mickala P.
Prevalence of haemoparasites in blood donors
attending the Regional Hospital Centre of
Franceville (South Gabon). J Infect Dis
Epidemiol 2022;8:270.
doi.org/10.23937/2474-3658/1510270
- Emch M, Root ED, Carrel M. Health and medical
geography. Guilford Publications. 2017.
- Anosike JC, Onwuliri Co. Études sur la
filariose dans l'État de Bauchi, Nigéria. II.
Prevalence of human filariasis in the Darazo
local government area. Application
Parasitol. 1994;35:242–250
- Pion SDS, Filipe Jan, Kamgno J, Gardon J,
Basañez MG, Boussinesq M. Microfilarial
distribution of Loa loa in the human
host: population dynamics and epidemiological
implications. Parasitology.
2006;133:101–109
- Adeoye GO, Akinsanya B, Otubanjo AO et al.
Prevalences of loiasis in Ondo state, Nigeria,
as evaluated by the rapid assessment procedure
for loiasis (RAPLOA). Annals of Tropical
Medicine & Parasitology.
2008;102(3):215-227. DOI:
10.1179/136485908X267867
- Scodellaro C. Perceived health among the
elderly: from medical criteria to practical
assessments. Retirement and Society.
2014;67(1):19-41
- Okonkwo ON, Hassan AO, Alarape T et al.
Removal of adult subconjunctival Loa loa
in Nigerian city dwellers. PLoS Neglected
Tropical Diseases. 2018;12(11):e0006920
- Ouedraogo NA, Korsaga-Some N, Traore F et al.
Loa loa filariasis in a tropical savanna
area: report of one case in Ouagadougou. Int
J Dermatol. 2020 Apr;59(4):482-483. doi:
10.1111/ijd.14782. Epub 2020 Jan 23. PMID:
31975376
- Dieki R, Eyang Assengone ER, Nsi Emvo E, Akue
JP. Profile of loiasis infection through
clinical and laboratory diagnostics: the
importance of biomarkers. Trans R Soc Trop
Med Hyg. 2023 May 2;117(5):349-357. doi:
10.1093/trstmh/trac116. PMID: 36520072; PMCID:
PMC10153730
|



















